|
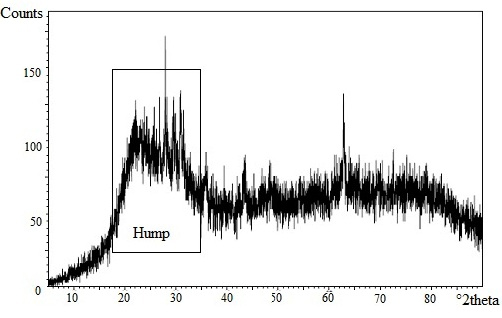
Introduction Candida albicans is a normal flora found in respiratory, gastrointestinal tract, mucous membranes, vagina, urethra, and skin. If the immune system becomes compromised, Candida albicans can infiltrate the bloodstream and diffuse to organs such as kidney, heart and brain [1]. Several diseases caused by Candida albicans are vulvaginistis candiduria, gastrointestinal candidiasis that can cause gastric ulcers and cancer [2]. Candida albicans forms biofilms due to an overdose of antifungal consumption [3]. Biofilms as a Candida albicans protection cause the body to exhibit toward immune system and antifungal agents. Biofilms can absorb the nutrients of the host cell therefore promote the growth colonies. The growth of biofilms is along with the increase of clinical infection in the host cell. Fungal infections can be treated with the proper use of antifungal agents. The use of antifungal agents should be accompanied by caution for the dangers of biofilm resistance. A new of antifungal substance is needed to prevent this effect. Therefore, It is necessary to conduct research to develop a new antifungal which is more effective against clinical disease mainly caused by bacteria, fungi or virus. One effort to develop new antifungal agents is the using of zeolite as an unique, cheap, and easy to synthesize material. Zeolite as a molecular sieves has been used for membranes, catalysts, and ion exchange [4-5]. Zeolite also has been used in the biomedical field as detoxifier, decontaminant, antibacterial, drug screen, biosensors and anti-tumour [6]. Zeolite clipnotilolite and zeolite A are widely used in the biomedical field [7-8]. Unfortunately, zeolite clipnotilolite as a zeolite has impurities such as montmorillonite, apatite, quartz, oxide of Ca, Al, Si, Fe, and other elements [9]. The presence of impurities can affect to the activity of zeolite and also impact the negative effects on health. On the contrary, zeolite A is an antifungal material that is safe, nonteratogenic agent, and it does not induce toxicity and carcinogenicity. The antifungal ability of zeolite A has been reported in previous studies [10-11]. The studies demonstrated that the activity of zeolite A against Acinetobacter junii with EC50 is 0.138 to 0.328 g/L, Saccaromyces cereviceae with EC50 is 2.88 to 5.47 g/L, Ceriodaphnia dubia with EC50 is 0.425 g/L[10]. Antifungal ability of zeolite A results generation hydroxyl ions during hydrolysis. The hydroxyl ions will increase the release of silicon and aluminium from zeolite framework and formed positively charged complex [AlmHnNapOqSi3-5]2+ or [(NaOH)x(AlO(OH))y (Si(OH)4)3-5]2+. The positively charged complex will interact with the electronegative cell walls of microbes (phosphoryl, carboxyl, and hydroxyl) through electrostatic interaction [10]. Meanwhile, silver is also an antifungal agent that is effective to inhibit the pathogenic microorganisms such as virus, bacteria and eukaryotic microorganisms [12]. The antifungal activity of silver is effective against about 650 types of bacteria. Silver ions can provide antifungal effects at low concentrations [13]. Submillimolar concentration of AgNO3 is lethal to gram-negative and gram-positive bacteria. In this paper, we present a preparation of zeolite A from bagasse ash, modification of preparation zeolite using silver, and investigation of the antifungal activity of silver loaded zeolite A against Candida albicans. This research also utilized the waste of sugar industry through the using of bagasse ash as a source of silica in the synthesis of zeolite A. Experimental Section Materials and Instruments Methods Synthesis of amorphous silica from bagasse Bagasse ash was placed into a beaker and poured with hot water. Then, concentrated HCl was poured on it and evaporated. This treatment was repeated three times. Furthermore, the mixture was poured into water and concentrated HCl (20:1 v/v) in water bath for 5 minutes. The mixture was then filtered and washed 4-5 times with hot water. The resulted powder was calcined at 3000C for 30 minutes, followed at 6000C for 1 hour to produce the amorphous silica. Sample was characterized by XRF to determine the concentration of silica. Synthesis of zeolite A from bagasse ash Synthesis of silver loaded zeolite A Study of antifungal activity of silver loaded zeolite A against Candida albicans Analysis of cell viability Candida albicans was suspended in 3 mL of phosphate buffer saline (PBS) and which was added with 50 mL of XTT solution and 8 mL of menadione. Then, it was incubated at 370C for 4 hours in the dark room and centrifugated at 2000 rpm for 15 minutes. The resulting supernatant was measured for absorbance using Spectrophotometer UV-Vis at 469 nm. Results & Discussion Amorphous Silica from Bagasse Ash
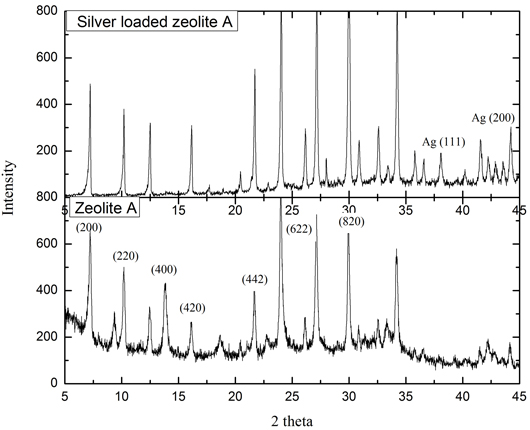
Silver Loaded Zeolite A from Bagasse Ash Silver loaded zeolite A was prepared by mixing zeolite A, and a solution of 0.05M, AgNO3. The ions of silver exchange the position of sodium ions in zeolite A framework. From the results of XRD analysis, it can be seen that there is a decreasing of peak intensity at 2θ 14.26; 24.87; 32.36; 35.49; 38.48; and 43.900. In the silver loaded-zeolite A, the diffractogram indicates the presence of silver that is proved by peak at 2θ 38.10 and 44.240. That peaks also represented the (111) and (200) hkl as Bragg’s reflection of face centered cubic crystalline silver. The pattern of diffractogram is similar with the previous report [22]. The addition amount of Ag in the zeolite A leads to a decrease in the amount of silica because the collapsed structure of zeolite. XRD pattern of zeolite A and silver loaded zeolite A from bagasse ash shown in Figure 2.
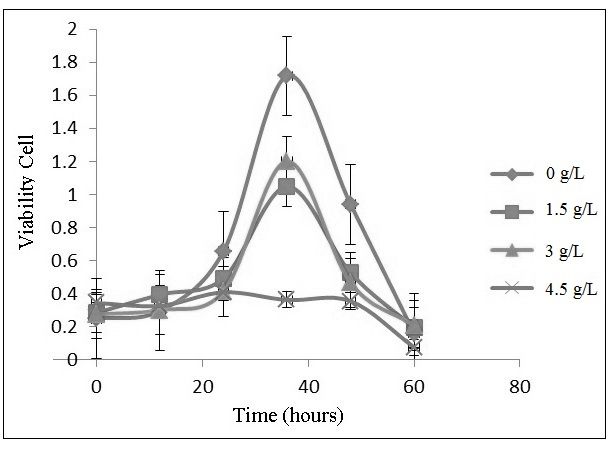
Antifungal Activity of Silver Loaded Zeolite A against Candida Albicans The influence of silver loaded zeolite A on the growth of Candida albicans is obtained through determination of cell viability using spectrophotometer UV-Vis at 469 nm. This analysis is based on the reducing ability of active cell metabolism towards XTT into a formazan orange with the presence of the dehydrogenase enzyme of mitochondria and menadione. The dehydrogenase enzyme of mitochondria is an oxidizing agent, thereby converting NADH into NAD+ and H+ by releasing electrons. Dehydrogenase enzyme consists of three kinds of complex enzymes (NADH-Q reductase, cytochrome reductase, and cytochrome oxidase). The presence of dehydrogenase enzyme of mitochondria can be an indicator of living cells. In contrast, dehydrogenase enzyme does not work in the death cell. XTT is not soluble in lipids so that it can not diffuse through the membrane. Therefore, in cell viability tests, menadione is needed as a media for the XTT to diffuses to mitochondria through cell membranes. Menadione acts as an electron carrier that is soluble in lipid. Dehydrogenase enzymes of mitochondrial reduced menadioneinto menadiol and generated NADH. Furthermore, menadiol diffuse out of the cell membrane and reacts with XTT, forms colored formazon compound [23-24]. The intensity of the colored formazan is analogue with the number of surviving cells. The profile of Candida albicans growth is influenced by the concentration of silver loaded zeolite A (0 to 4.5 g/L), as shown in Figure 3. The growth profile consists of four phases: lag, exponential, and a death phase. Lag phase is an adaptation phase that occur when the population of Candida albicans is inoculated into a new medium. Exponential phase is a phase which at Candida albicans reproduces by itself. Death phase (lysis) is a phase of decreasing the number of Candida albicans due to nutritional deficiencies or unsupported medium. There are three phases of growth profile in the control media (0 g/L) and in the concentration of silver loaded zeolite A 1.5 and 3 g/L. They are lag exponential, and death phase. Lag phase occurs in the 0 to 12 hours, whereas exponential phase occurs at 12 to 36 hours, and death phase occurs after 36 hours. In the concentration of silver loaded zeolite A 4.5 g/L, one phases of growth profile was obtained, death phase. The formation of biofilm was occured at 48 and 60 hours in the control medium (0 g/L).
The graph indicates that the silver loaded zeolite A is able to inhibit the growth of Candida albicans. The greater the concentration of silver loaded zeolite A, the larger inhibitory activity against Candida albicans. The mechanism of inhibition is due to the releasing of silver ions from zeolite A. Table 1 presents the amount of silver ion that release from zeolite framework. The silver ion inhibited cellular respiration system and binded with sulfyhydrals enzyme, therefore destroyed the protein bonds [25-27]. Therefore, the optical densityis decreased, the amount of the growth of Candida albicans should also be decreased.
From the results of antifungal activity of silver loaded zeolite A with various concentration, it concluded that silver loaded zeolite A with a concentration of 4.5 g/L is an optimum concentration to inhibit the growth of Candida albicans. In this condition, the concentration of silver is 1.8 mg/L. Meanwhile, the occupational exposure limit for silver ion is 10 mg/L [28]. Therefore, silver loaded zeolite A potential to apply in manufacturing of medicine, medical devices, household items, and textiles where antifungal properties are required. Despite of the mechanism of silver ions releasing, the inhibition mechanism Candida albicans is also due to hydroxyl ions that are produced by zeolite A due to hydrolysis, resulting higher pH. Hrenovic et al. [10] has studied this phenomenon. The possible reaction of generating of hydroxyl ions from zeolite is:
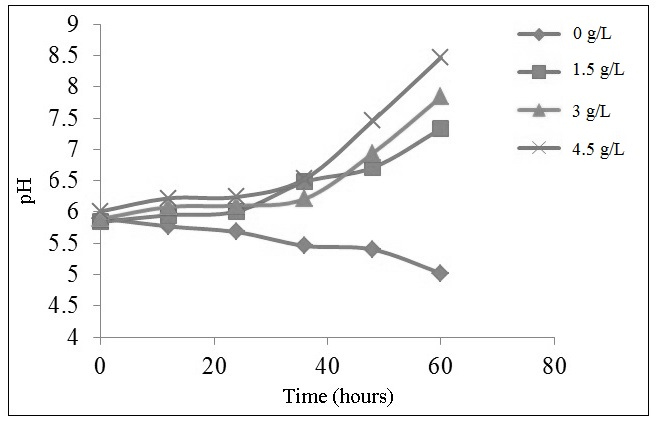
In this research, the pH of water is increased respectively 7.33 to 5.84 on zeolite concentration of 1.5 g/L; 5.89 becomes 7.84 on zeolite concentration of 3 g/L; and 6.01 to 8.47 at a concentration of zeolite 4.5 g/L. The greater the silver loaded zeolite A concentration, the higher the media basicity (Figure 4). According to the previous research [10], the hydroxyl ions will increase the release of silicon and aluminum from the zeolite framework forming positively charged complex [AlmHnNapOqSi3-5]2+ or [(NaOH)x(AlO(OH))y (Si(OH)4)3-5]2+. When Candida albicans is planktonic, the positively charged complex will interact with the electronegative cell membrane of microbes, such as phosphoryl, carboxyl, and hydroxyl through electrostatic forces.
Moreover, the alkalinity is not a major factor in inhibiting the growth of Candida albicans. At 36 hours, all of media generate a similar pH but different value of the cell viability. It can be concluded that the amount of released silver ions has a more substantive role than hydroxyl ions to inhibite the growth of Candida albicans. Conclusion This study successfully synthesized silver loaded zeolite A from bagasse through ion exchange mechanism. Silver loaded zeolite A has antifungal activity against Candida albicans through the release mechanism of silver ions. The optimum concentration of silver loaded zeolite A in inhibiting the growth of Candida albicans is 4.5 g/L at the optimum time of 60 hours. The use of silver loaded zeolite A with this concentration is appropriate for the use in manufacturing of medicine, medical devices and other industries that required the antifungal properties. Acknowledgment The authors would like to show appreciation to the Department of Chemistry, Faculty of Science and Technology, Universitas Airlangga for the use of laboratory facilities. The authors would also like to express gratitude to Prof. Dr. Afaf Baktir, MS for the guidance during the research. References
|
||||||||||||||||||||