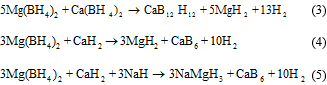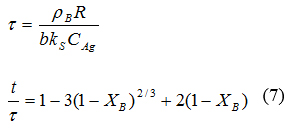|
Introduction The desire to have a clean atmosphere has led to an increased urge to develop alternative sources of energy. The greatest challenge with the use of hydrogen as a source of fuel is the unavailability of suitable storage mechanisms. For solid state hydrogen storage in automobiles, complex metal hydrides that are lightweight, low cost, and high in hydrogen density are potential candidates [1,2]. The use of Mg(BH4)2 as a potential storage material has been gaining popularity in recent times due to its high gravimetric hydrogen content of 14.9 wt% [3-11]. The hydrogen, however, cannot be easily harnessed due to thermodynamic and kinetic barriers. Studies have shown that in complete dehydrogenation, Mg(BH4)2 should produce MgB2 and 4 moles of hydrogen gas, according to equation (1) [12,13]. A popular way of improving the hydrogen storage properties of hydride compounds, first developed by Reilly and Wiswall [14], is mixing/destabilizing with other additives. Vajo et al. [15,16] showed that addition of MgH2to LiBH4 reduces its desorption temperature by changing the reaction pathway. The mixture also became reversible under moderate conditions. Similarly, a mixture of CaH2 and LiBH4 was predicted by Alapati et al. [17,18] to release 11.7 wt% of hydrogen, according to equation (2). They also calculated the enthalpy of the reaction to be 47 kJ/mol H2. Studies showed that the system released hydrogen at a lower temperature than pure LiBH4 [19-24]. Pinkerton and Meyer [21] reported that samples of 6LiBH4 + CaH2 + 0.25 TiCl3, prepared by ball milling, released about 9.1 wt% of hydrogen during a TGA scan and that samples were successfully rehydrogenated under 83 bar H2 and 4000C. Severa et al. [8] found that it was possible to reversibly store about 11 wt% hydrogen in Mg(BH4)2. This was achieved under extreme conditions by direct hydrogenation of MgB2 at a pressure of 900 bar and a temperature of 4000C. The addition of catalyst/additives has also been reported to improve the hydrogen storage properties of Mg(BH4)2 [25 - 28]. For instance, Yang et al. [26] reported the reduction of the onset temperature for hydrogen release to 1500C by the addition of LiH. It was also shown by Bardaji et al. [12] that a composite mixture of LiBH4 and Mg(BH4)2 releases about 7 wt% of hydrogen at lower temperature than that of the individual borohydrides. A theoretical study by Ozolin et al. [29] using DFT calculations identified some reaction schemes that have the potential of reversibly storing hydrogen. Some of these reactions schemes that have been studied by our group are highlighted in equations (3)-(5).
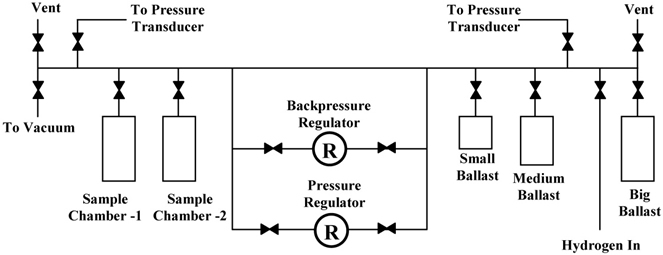
In previous studies, we determined the hydrogen properties of these systems [10]. We also found that the mixture of Mg(BH4)2 and Ca(BH4)2 releases hydrogen at a faster rate than either of the components [11]. In this present study, we present the kinetics and modeling studies of hydrogen desorption of systems in reaction schemes (4) and (5). Experimental CaH2 and NaH were obtained from Sigma-Aldrich and were used without further treatment. Unsolvated Mg(BH4)2 was prepared from MgH2 and triethylamine borane, as described by Chłopek et al. [9]. All sample preparations were carried out in an argon-filled Vacuum Atmospheres glove box with oxygen and moisture levels below 1 ppm. The sample mixtures were ball-milled for 10 h using a SPEX 8000D mixer/mill. PCT isotherms were determined for each sample using an Advanced Materials Corporation Gas Reaction Controller PCI unit. This unit was fully automated and was controlled by a Lab View-based software program. Kinetics measurements were done in an all stainless steel Sieverts apparatus. This apparatus is described in Fig. 1. It contained a back pressure regulator for controlling the pressure that was applied to the sample. Pressure transducers were used to monitor the pressure in the reservoirs and the sample reactor. Kinetics measurements were done at the same temperature and thermodynamic driving force. The thermodynamic driving force, denoted as the N-Value, is defined as the ratio of the mid plateau pressure, Pm, to the opposing pressure, Popp (i.e., N=Pm/Popp). The experiments were all carried out at 4500C and N =10. Further details about the technique of constant pressure thermodynamic forces are published elsewhere [10, 11, 23, 24, 30, 31].
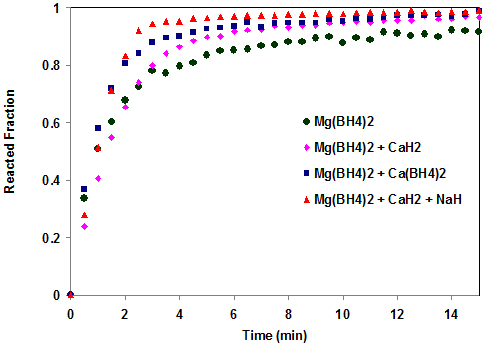
Results & Discussions Desorption kinetics of the Mg(BH4)2/CaH2 and Mg(BH4)2/CaH2/NaH mixtures as well as that of the pure Mg(BH4)2 components were performed at 4500C (Fig. 2). These measurements were carried out at a constant pressure thermodynamic driving force of N=10. In order to accomplish this, it was first necessary to determine a PCT isotherm for each sample. The PCT isotherms of Mg(BH4)2 and the mixtures at 4500C have been reported [10,30]. The opposing pressures determined for the mixtures from the isotherms are shown in Table 1.
All of the reactions are faster than that of pure Mg(BH4)2. All of the reactions except Mg(BH4)2, reached 90% completion on or before 5 minutes. Table 1 shows the time taken for the hydrogen desorption reactions to reach 90% completion (T90). It takes about 12 min for the pure Mg(BH4)2 to reach T90, while the mixtures take between 2.5 - 4.0 mins to reach 90% completion. All the mixtures have also been reported to have higher desorption temperatures than the pure Mg(BH4)2, except the Mg(BH4)2/CaH2/NaH mixture [30]. Modeling studies were done to determine the process controlling the rates of hydrogen desorption from the systems. A set of equations used by Smith and Goudy [31] in modeling the LaNi5-xCox hydride system were used. The models were used to determine whether the hydrogen desorption process is controlled by a reaction occurring at phase boundary or hydrogen diffusion. Where
Where
The various terms in the equations are defined as follows: t = time at a specific point in the reaction; XB = fraction of the material reacted; R = initial radius of the sample particles; b = stoichiometric coefficient of the material; CAg = the gas phase concentration of the reactant; De = the effective diffusivity of the hydrogen atoms in the material; ρβ = the density of the material; and ks = a rate constant. In the modeling studies, the experimental kinetics data of the systems were statistically fitted with theoretically generated data based on equations (6) and (7).
Values of τ were selected that minimized the difference between the experimental and theoretical curves. The parameters (R, b, De, ρβ , and ks) were not used to determine τ in the equations and we don't have their values. Tau (τ) is essentially a fitting parameter. The values of τ for each reaction were determined statistically by minimizing the standard deviation between the experimental and theoretical curves. Equation (6) corresponds to a situation in which the rate is controlled by diffusion, while equation (7) is that of a process where the rate is controlled by reaction at the phase boundary.
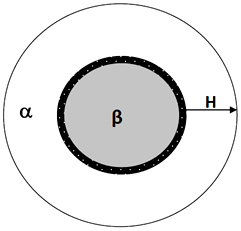
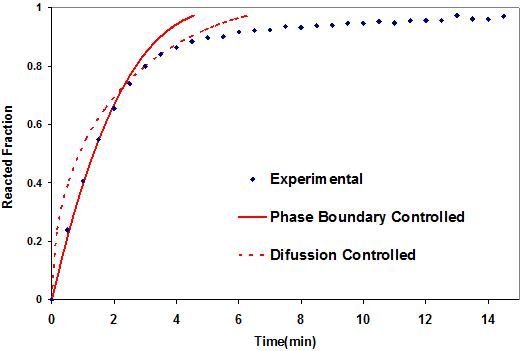
Equations (6) and (7) are based on a shrinking core model in which desorption begins from a fully hydrided sphere. As hydrogen desorbs from the particle, an outer layer of dehydrided material begins to cover the sphere. The outer dehydrided layer grows as hydrogen desorption continues and the inner sphere shrinks. A phase boundary separates the outer and inner spheres. A schematic of this shrinking core model is shown in Fig. 3. Hydrogen desorption occurs at the phase boundary and hydrogen atoms diffuse through the outer dehydrided layer. The two processes occur simultaneously throughout the entire desorption process. The kinetics may be limited by the rate of reaction at the phase boundary or by the rate of hydrogen diffusion through the outer dehydrided layer, depending on which is slower. The plot in Fig. 4 for Mg(BH4)2/CaH2 shows that the phase boundary controlled model fits the experimental curve up to 70% of the reaction, while the diffusion controlled model fits between 75 and 85%. Above that region, none of the two models fits. Therefore, the system rate determining step changes during the course of the reaction. The results correspond to those reported earlier for the Mg(BH4)2/Ca(BH4)2 system [11]. The CaH2 changed the rate determining process of the mixture from that of pure Mg(BH4)2 controlled by the diffusion process [11].
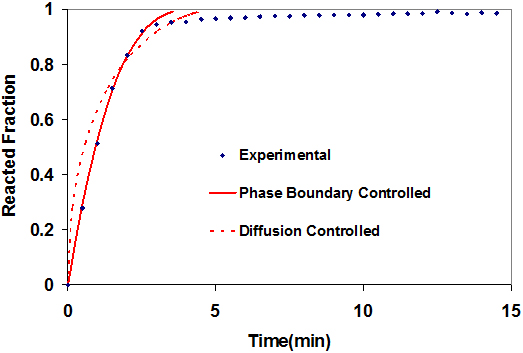
The modeling curve of the Mg(BH4)2/CaH2/NaH mixture is shown in Fig. 5. Model fitting for the Mg(BH4)2/CaH2/NaH mixture shows a good fit of the phase boundary controlled model with the experimental up to about 90% of the reaction. The diffusion controlled model does not fit at all for this process. Therefore, the rate limiting process in the dehydrogenation of Mg(BH4)2/CaH2/NaH system is a reaction at the phase boundary. It can be seen that the mixture containing NaH released hydrogen at the fastest rate of any system under study. It may be that the Na+ enhances the rate of hydrogen diffusion in the system, thereby resulting in an increase in the overall reaction kinetics. The high desorption temperature of Mg(BH4)2/CaH2/NaH, the highest of the three mixtures involving Mg(BH4)2, can be attributed to the high reactivity of the Na in the system [30].
Conclusion The studies have shown that the rate of hydrogen desorption from Mg(BH4)2 can be increased by destabilization. The mechanical alloying of Mg(BH4)2 with CaH2 and CaH2/NaH improved the rate of hydrogen desorption, with the ternary mixture having the fastest kinetics. Modeling studies also show that the rate of hydrogen desorption from Mg(BH4)2/CaH2 is controlled by reaction at the phase boundary in first 75% of the reaction, and changed to diffusion in 75-85% of the reaction. Furthermore, hydrogen desorption from Acknowledgments This research was financially supported by the U.S. Department of Energy Grant Number DE-FC36-06GO86046 and the U.S. Department of Transportation Grant Number DTOS59-07-G-00056. The authors thank Dr. Andrew Goudy for providing the instrumentation used in this research. References
|
||||||||||||||||||||||||||||||||||||||||||