|
Introduction Complex metal hydrides with high gravimetric hydrogen content such as amides, alanates, and borohydrides are being considered as potential candidates for hydrogen storage [1-6]. Notable among the challenges of using borohydrides as hydrogen storage materials are their high dehydrogenation temperatures, diborane production, poor reversibility, as well as cycle life [6-11]. The study of Mg(BH4)2 as a potential hydrogen storage material is gaining more attention due to its light weight and high gravimetric hydrogen content. Konoplev and Bakulani reported some of its properties in 1971 [12]. Theoretical studies predict MgB12H12 and MgB2 to be the only energetically stable and possible decomposition products of Mg(BH4)2 [13]. Thermal analysis showed that most of the hydrogen loss from the decomposition of Mg(BH4)2 occurs between 300 to 4000C [14]. Decomposition started at 2900C and about 13 wt% hydrogen was released up to 5000C with a minimal trace of B2H6. At 2900C, MgH2, MgB2, and Mg were observed while at 4500C and above, Mg, MgB2, and an unknown phase were detected. The effect of ball milling and several additives on the decomposition of Mg(BH4)2 have been investigated [15-29]. Ball milling does not have any effect on the decomposition temperature [15-16]. The sample becomes amorphous during ball milling but is recrystallized to the α-phase after heating to about 1500C. Li et al. reported the reduction of the onset desorption temperature of Mg(BH4)2 from 2620C to 880C by addition of TiCl3 [16]. They proposed that the improvement is due to the formation of more stable TiB2 or less stable MgTi-based borohydride. Badaji et al. also investigated Mg(BH4)2 mechano-chemically alloyed with several metal chlorides [15] and found that combined Nb-Ti additives lowered the onset temperature by up to 1250C. However, none of the additives were able to improve the reversibility of the material. There have been reports on studies involving destabilization of Mg(BH4)2 with hydrides, borohydrides, and amides [20-26]. Studies have shown that the system consisting of Mg(BH4)2 and LiBH4 possess superior hydrogen storage properties to either of the constituents [20-22]. Similarly, Temperature Programmed Desorption, TPD, analyses have shown that the desorption temperature of Mg(BH4)2 can be lowered by ball milling with Ca(BH4)2 [26]. The resulting mixture absorbed and released hydrogen with a well-defined plateau region in the pressure composition isotherm (PCI). This mixture has also been reported to release hydrogen at a faster rate [27]. In the present study, the effect of NaH, silicon, and carbon on the hydrogen storage properties of Mg(BH4)2 is presented. Experimental The materials used in this research were obtained from Sigma Aldrich; they were hydrogen storage grade and were hence used without further purification. The Mg(BH4)2 was prepared from MgH2 and triethylamineborane, as described by Chłopek et al. [14]. All sample handling, weighing, and loading were performed in a Vacuum Atmospheres argon-filled glove box with oxygen and moisture levels below 1 ppm, to prevent contamination from air and moisture. The glove box was vacuum cleaned several times using purified argon gas to remove air and moisture. The mixture of the samples were mechanically mixed for 10 hrs using a SPEX 8000M Mixer Mill that had a stainless steel milling pot, which contained four small and two medium sized stainless steel balls. X-ray powder diffraction, XRD, analysis was used to determine whether new phases were formed by milling the different substances. A Panalytical X’pert Pro MPD Analytical X-ray Diffractometer Model PW 3040 Pro was used for these analyses. The samples were covered with a Kapton film to protect them from air and moisture. Temperature Programmed Desorption, TPD, was done in a gas reaction controller-PCI unit to evaluate the hydrogen desorption properties of each reaction mixture. This apparatus was manufactured by the Advanced Materials Corporation. The unit was fully automated and was controlled by a Lab View-based software program. An in-situ XRD analysis was also carried on the mixture at different temperatures to determine the species that were present during the dehydrogenation of the mixtures. Results & Discussion
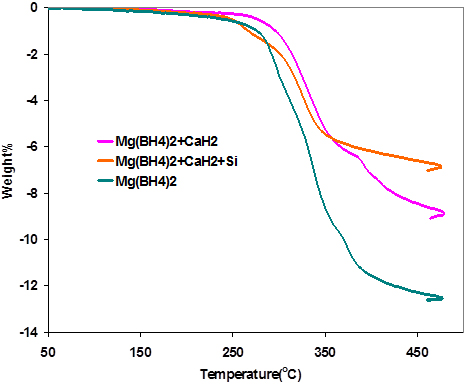
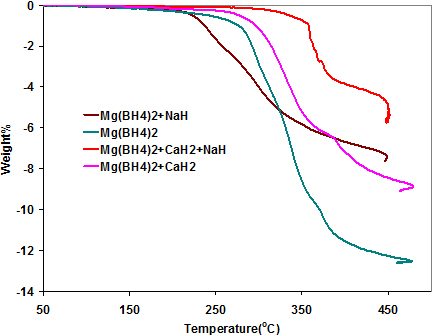
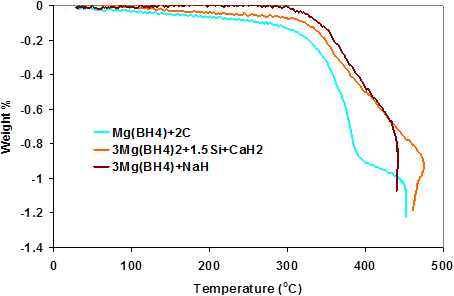
The TPD curve of Mg(BH4)2/CaH2/Si is shown in Fig. 1 along with those of pure Mg(BH4)2 and Mg(BH4)2/CaH2. It has been shown that the decomposition temperature of hydrides could be lowered by alloying with silicon [28, 29]. The addition of silicon reduced the onset temperature of the Mg(BH4)2/CaH2 mixture, but the overall decomposition temperature is still higher than that of Mg(BH4)2. The silicon also reduced the total amount of hydrogen desorbed by increasing the overall weight of the mixture.
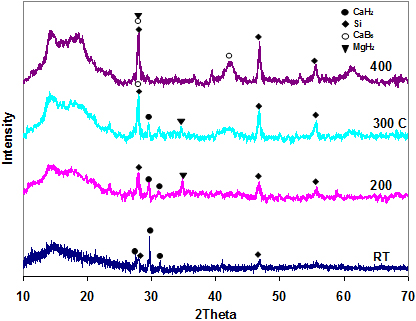
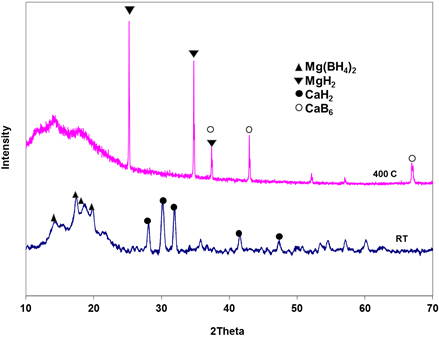
From the results of the XRD studies shown in Fig. 2, it is clear that the silicon did not form any alloy with CaH2. It only remains in the mixture throughout the dehydrogenation reaction. The expected product of dehydrogenation of this mixture, Mg2Si according to equation 1, as predicted by Alapati et al.[30] and Ozolin et al. [31] is conspicuously absent in the XRD pattern. The products of the dehydrogenation are also similar to those of the mixture involving only Mg(BH4)2 and CaH2 (i.e., MgH2 and CaB6 shown in Fig. 3).
In Fig. 4, the TPD curve of Mg(BH4)2/NaH shows a big reduction in the onset temperature of Mg(BH4)2, releasing about 6 wt% of hydrogen below 3500C. This shows that NaH is better at destabilizing the Mg(BH4)2 than either CaH2 or a mixture of CaH2 and NaH.
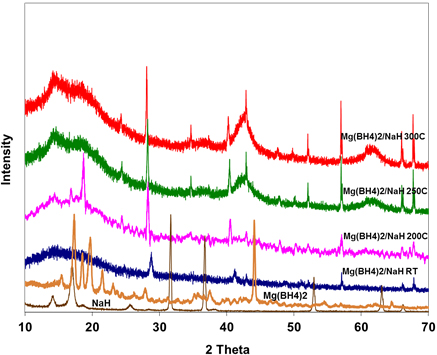
In the XRD analysis of the Mg(BH4)2/NaH shown in Fig. 5, neither the peaks of Mg(BH4)2 nor NaH were present in the as-milled sample at room temperature. This suggests that a new species might have been formed during ball milling. The appearance of some new peaks, which become persistent even after heating to 3000C, indicates the presence of a new phase.
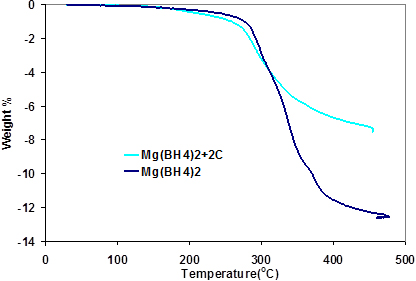
Fig. 6 contains the TPD curve of the Mg(BH4)2/C system alongside that of pure Mg(BH4)2. It can be deduced from the curve that the presence of carbon does not affect the desorption temperature of the mixture. However, the amount of hydrogen released was reduced and this can be ascribed to the physical presence of carbon adding to the overall weight of the mixture. Further evidence from of the XRD analyses shows that carbon is just passive in the mixture.
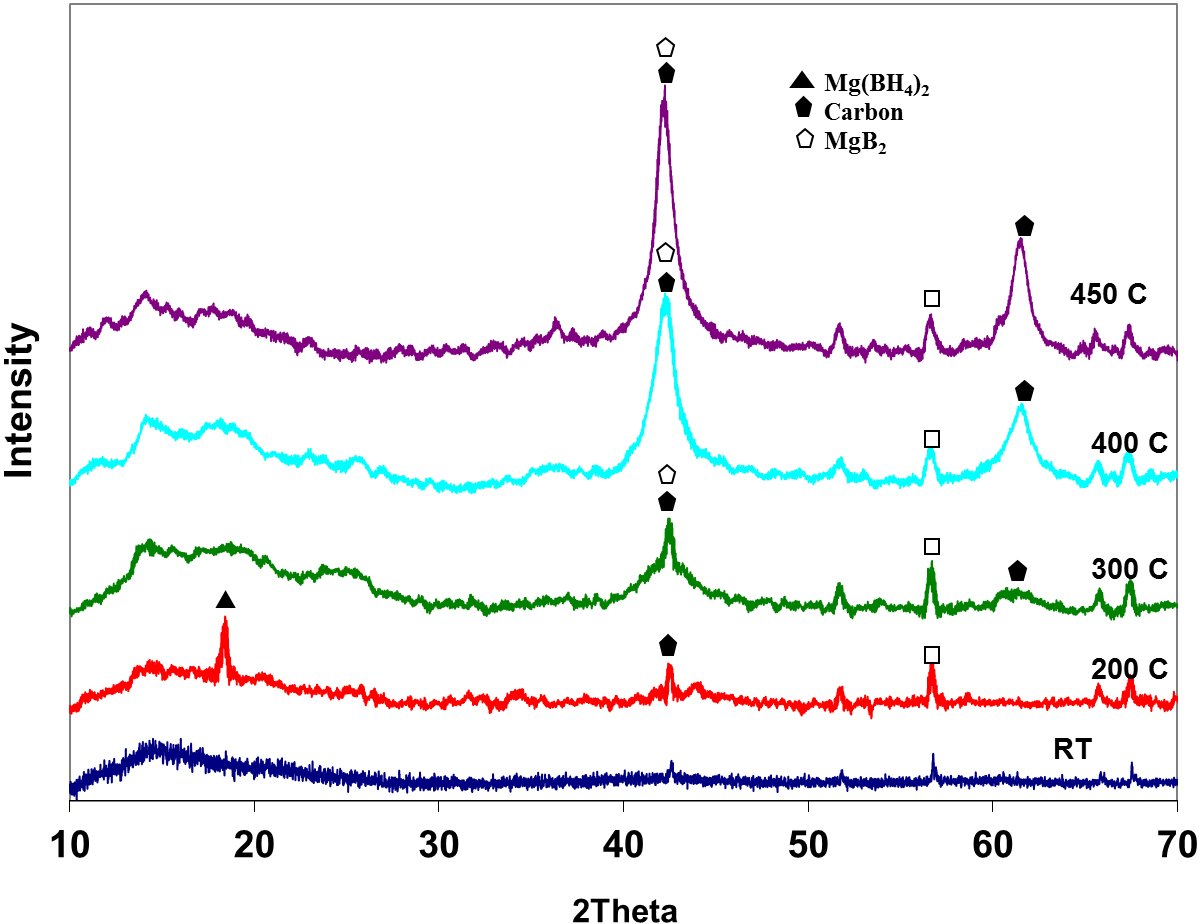
In Fig. 7, the major peaks of carbon were persistent throughout the analysis. No peak for the expected product of dehydrogenation (MgB2C2) according to equation 2 was detected. MgB2 is the only species identified as the product after dehydrogenation.
The TPD curves of the rehydrogenated samples are shown in Fig 8. All the mixtures released just about 1 wt% hydrogen in the second dehydrogenation cycle, showing that reversibility of these mixtures are very poor. Attempts to obtain the isotherms for the mixtures were unsuccessful as no clear plateau region was observed, also indicating that their reversibility is poor.
Conclusion The results from this study have shown the effects of various additives on the dehydrogenation of borohydrides. Of all the various ball milled mixtures of Mg(BH4)2 and additives, NaH is the only one that effectively lowers the desorption temperature of Mg(BH4)2. This may be due to the formation of new borohydride species during ball-milling of the mixture. Other additives did not have any effect on the desorption temperatures, but rather reduced the amount of hydrogen released due to their physical presence increasing overall weight of the mixture. The mixtures also showed poor reversibility, releasing much lower amounts of hydrogen in the second dehydrogenation. Acknowledgments This research was financially supported by grants from the U.S. Department of Energy Grant and the U.S. Department of Transportation. The authors specially thank Professor Andrew Goudy for providing the equipment used in this research. References
|