|
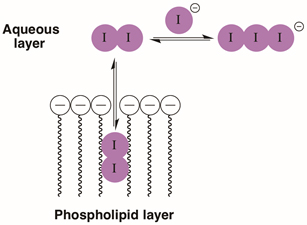
Iodine is a useful topical antiseptic due in part to its ability to dissolve in both hydrophobic and hydrophilic media. It has broad spectrum antimicrobial activity and its ability to readily pass through cell membranes plays an important role [1]. Iodine is also a useful catalyst in carbohydrate chemistry for a range of transformations [2]. Using it in such a capacity certainly makes traditional synthetic chemistry more colorful, as solutions of iodine are intensely colored, ranging from blue to violet to deep red depending on the solvent and other solutes. Iodine can be readily converted to colorless iodide solutions by the addition of sodium thiosulfate or sodium metabisulfite. Because iodine can pass between an organic/aqueous interface (Figure 1), this property offers an obvious visual demonstration of membrane permeability that is related to the distribution of molecules in living organisms. This simple protocol can be carried out using readily available household chemicals. Commercially, iodine solutions are often sold in a complex with the polymer povidone as a topical antiseptic. Iodine can be dissolved in an aqueous solution by adding iodide salts to form the I3- anion (Figure 1); this is also used to make a tincture of iodine. When an aqueous solution of iodine/iodide or iodine-HI-povidone complex is partitioned with an organic solvent such as ethyl acetate or cyclohexane, coloration of the organic layer occurs almost immediately (see test tube 1, Figure 2). This results from free iodine passing into the organic solvent and eventually the top layer will achieve a steady level of coloration. Once this has occurred, a reducing agent such as sodium metabisulfite can be added. This will dissolve only in the aqueous layer and quickly reduce iodine to iodide, removing color from the bottom layer (see test tube 2, Figure 2). Still, a small cloud of iodine color can be seen settling back into the bottom layer as iodine passes back into the aqueous layer from the organic layer. If this bilayer is mixed for extended periods of time, eventually all color from the iodine will be removed if enough metabisulfite is used. This offers a simple look at the partitioning of iodine and its removal using an aqueous based reaction. Each stage demonstrates iodine’s ability to dissolve in both organic and aqueous media, while using chemistry that takes place only in the water layer makes this more obvious.
Details of Demonstration Sourcing components:
Method shown in Figure 2: Approximately 2 mL of commercial iodine solution was diluted with an equal volume of distilled water and ca. 4 mL of ethyl acetate was added by pipette, which was also used to mix the two layers. The povidone-iodine solution should be diluted with water, as it often contains a mixture of ethanol and water which can interfere with partitioning. [Note: cyclohexane offers a more vibrant color than ethyl acetate, but is less commonly available.] Once steady color in the organic layer was obtained, 50 mg sodium metabisulfite was added with stirring to facilitate reduction. Vigorous mixing will remove color from both layers, while avoiding any agitation after the addition of metabisulfite will allow for a very slow dissipation of iodine color. References
|