|
Introduction Chemical equations are widely used in theory and practice. It is well known that the elements and compounds are expressed by their symbols and formulae, respectively. The chemists have devised a shorthand method to represent a chemical reaction symbolically, instead of in words. Such a shorthand method of representation of a chemical reaction with the help of symbols and formulae is called a chemical equation. If both the qualitative, as well as the quantitative, aspects of the reaction are reflected by a chemical equation, the equation is said to be a true, or correct, or balanced chemical equation. On the other hand, if a chemical equation only reflects the qualitative aspect of the chemical reaction, the equation is said to be an unbalanced chemical equation. A balanced chemical equation also helps in forming a mental picture in regard to the physical nature and properties of the reactants (viz., colour, state, etc.), as well as the chemical result and its physical nature. Balancing of chemical equations is an essential part of chemical mathematics called Stoichiometry, that is concerned with the mass and mole relationships determined by chemical equations and formulae. For these reasons, writing down a chemical equation correctly has drawn much attention in Chemical Education Research [1,13]. Chemistry students ought to be well conversant in writing and balancing chemical equations. The balancing of chemical equations is also an excellent exercise from demonstrative as well as pedagogical points of view. This paper concentrates on the algebraic method of balancing chemical equations. The usual procedure of balancing chemical equations in the algebraic method is based on the fundamental principles of conservation of mass and charge. According to the principle of conservation of mass, the quantity of each element must remain the same on both sides in a chemical reaction. Thus, the same quantity of each element must appear on both sides of the true or balanced chemical equation. Also, from the principle of conservation of charge, the charge must be conserved in a chemical reaction. So, the same charge must remain present on both sides of the balanced chemical equation. Balanced equations are to be written with the smallest integer coefficients. If the coefficient is 1, it is understood. The underlying principle as well as the procedure of the algebraic method of balancing chemical equation has been explained first in a novel way, considering a few examples. After applying this procedure to typical chemical equations, it is possible to generate an infinite number of independent balanced forms for each of those chemical equations without inviting any kind of ambiguity. Thus, the present work enhancing the efficiency of the algebraic method of balancing chemical equations deserves special attention to the scientific and engineering community. Basic Principles Involved in the Algebraic Method of Balancing Chemical Equations Let us consider the chemical equation: R1 + R2 + R3 + … + Rm → P1 + P2 + P3 + … + Pn … (1) with “m” number of reactants and “n” number of products. Let the number of different kinds of elements on each side of this chemical equation be “s”. The balanced form of this chemical equation may be written as: ( c1 ) R1 + ( c2 ) R2 + ( c3 ) R3 + … + ( cm )Rm → (Cm+1)P1 + (Cm+2)P2 + (Cm+3)P3 + … + (Cm+n)Pn… (2) where the (m + n) coefficients c1, c2, c3, … , cm, cm+1, cm+2, cm+3, … , cm+n are smallest possible positive integers. Since equation (2) is a balanced form of the chemical equation, the total number of each kind of the “s” number of elements on both sides of equation (2) must be equal. Equating the total number of each kind of “s” elements on both sides of equation (2), it would then be possible to write down a total of “s” linear equations involving the coefficients c1, c2, c3, … , cm, cm+1, cm+2, cm+3,... , cm+n. It may now be noted that each of the aforesaid “s’ number of equations will remain unchanged if both sides of each of those “s” number of equations are multiplied by µ (where µ is a constant or a positive integer or a non-negative proper fraction). Thus the aforesaid “s” equations will then be changed to “s” number of other linear equations involving the coefficients x1, x2, x3, … , xm, xm+1, xm+2, xm+3, … , xm+ n, where x1 = µc1, x2 = µc2, x3 = µc3, … , xm = µcm, x m+1 = µ m+1 , x m+2 = µ m+2, x m+3 = µ m+3, … , x m+ n = µ m+n. It may further be noted that: x1 : x2 : x3 : … : xm : xm+1 : xm+2 : xm+3 : … : xm+ n = c1 : c2 : c3 : … : cm : cm+1 : cm+2 : cm+3 : … : cm+ n, Thus, to find the balanced form of a chemical equation of the type represented by equation (1) above, we should consider the “s” number of linear equations involving the (m + n) coefficients, x1, x2, x3, …, xm, xm+1, xm+2, xm+3, … , xm+ n, where xi= µci , i ε { 1, 2, 3, … , m, m + 1, m + 2, m + 3, … , m + n }. Those “s” number of equations are to be employed to ultimately find the ratio x1 : x2 : x3 : … : xm : xm+1 : xm+2 : xm+3 : … : xm+n in the simplest possible positive integer form, c1 : c2 : c3 : … : cm : cm+1 : cm+2 : cm+3 : … : cm+ n, with a view to identifying the required coefficients, c1, c2, c3, … , cm, cm+1, cm+2, cm+3, … , cm+ n, with the help of which the balanced form(s) of the chemical equation under consideration could be easily written. If the chemical equation under consideration contains ions, one more linear equation that will result from the conservation of charge is to be considered. In such a case there will be in all (s +1) equations involving the coefficients xi s, where xi = µci, i ε { 1, 2, 3, … , m, m + 1, m + 2, m + 3, … , m + n }. Those (s +1) equations will then be considered as above to pick out the required coefficients, c1, c2, c3, … , cm, cm+1, cm+2, cm+3, … , cm+n, in order to write down the balanced form(s) of such a chemical equation. The Novel General Procedure of Balancing Chemical Equations by Algebraic Method The novel general procedure of balancing chemical reaction equation by algebraic method to be considered in this paper is based on the principle of conservation of mass as well as the principle of conservation of charge like the traditional schemes. The procedure of balancing a chemical equation in the novel approach is based on the following steps. Step 1: Write the chemical reaction equation using the coefficients xi s, where i ε { 1, 2, 3, 4, 5, 6, 7, … , m + n}, “m” and “n” being respectively the total number of reactants and that of the products of the relevant chemical reaction. Step 2: Form a set of “s” linear equations involving the coefficients xi s by equating the total number of element of each kind on both sides of the chemical equation, where “s” is the total number of different kinds of elements on each side of the chemical equation under consideration. Step 3: If charged molecules or/and ions remain present in the chemical equation, form the (s + 1)th linear equation involving the coefficients xi s by equating the total charge on both sides of the chemical reaction equation. Step 4: Consider all the equations formed in Step 2 and Step 3. Step 5: By choosing any one of the coefficients xi to be equal to k1, where k1 is a positive integer or a non-negative proper fraction, see whether it is possible to express all other remaining coefficients xi s in terms of k1. If so, continue on to Step 6. Otherwise, go to Step 8. Step 6: Express the ratio x1 : x2 : x3 : … : xm+n in the simplest possible positive integer form c1 : c2 : c3 : … : cm+ n, and identify the corresponding values of the coefficients ci s , i ε { 1, 2, 3, … , m + n }. Step 7: Use the values of the coefficients, c1, c2, c3, … , cm+n obtained in Step 6 to write down the only one balanced form of the relevant chemical equation. Step 8: If by choosing the value of a particular coefficient xi to be equal to k1, where k1 is a positive integer or a non-negative proper fraction, it is not possible to express all other remaining coefficients xi s in terms of k1, choose a second coefficient xi to be equal to k2, where k2 is a positive integer or a non-negative proper fraction, and see whether the remaining (m + n − 2) coefficients xi s can be expressed in terms of k1 and k2. If so, continue on to Step 9. Otherwise, go to Step 13. Step 9: Remembering that each of k1 and k2 may be a positive integer or a non-negative proper fraction, assign any one of such pair of values to k1 and k2 out of the infinite number of choices and find the values of all the coefficients xi s, i ε { 1, 2, 3, … , m + n }. Step 10: Express the ratio x1 : x2 : x3 : … : xm+n in the simplest possible positive integer form, c1 : c2 : c3 : … : cm+n, and identify the corresponding values of the coefficients ci s, i ε { 1, 2, 3, … , m + n }. Step 11: Use the values of the coefficients, c1, c2, c3, … , cm+n obtained in Step 10 to write down one balanced form of the relevant chemical equation for the particular choice of the values of k1 and k2 as made in Step 9. Step 12: Repeat Step 9 to Step 11 for each and every other pair of values of k1 and k2 out of an infinite number of choices to derive the corresponding other independent balanced forms of the chemical equation under consideration. Step 13: Repeat Step 8 to Step 12 by choosing a third, fourth, fifth, etc. coefficients xi s respectively as k3, k4, k5, etc., where ki is a positive integer or a non-negative proper fraction, i ε { 3, 4, 5, … , m + n }, to obtain the infinite number of independent balanced forms of the chemical equation. Application of the Novel General Procedure of Balancing Chemical Equations by Algebraic Method In this section, the novel general procedure of balancing chemical equations by algebraic method will be employed to illustrate the said procedure by considering some examples. Example 1: Let us consider the balancing of the chemical equation: Let the balanced equation be: ( x1 ) NaNO3 + ( x2 ) Zn + ( x3 ) NaOH → ( x4 ) Na2ZnO2 + ( x5 ) NH3 + ( x6 ) H2O where the coefficients xi s, i ε { 1, 2, 3, 4, 5, 6 } are smallest positive integers. Equating the number of atoms of each element on both sides of the above chemical equation we then obtain the following equations.
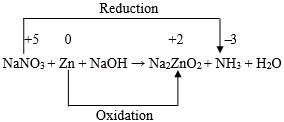
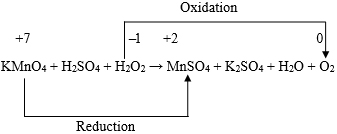
For N: x1 = x5 … (4) For O: 3x1 + x3 = 2x4 + x6 … (5) For Zn: x2 = x4 … (6) For H: x3 = 3x5 + 2x6 … (7) Thus, we have five equations and the number of unknown integers to be found out is six. In order to evaluate the values of the six unknown positive integers in smallest form with the help of the aforesaid five equations, we now proceed as follows. Let x1 = k. Then from equation (4), we get, x5 = k. Hence from equations (3), (5), (6) and (7) we respectively get, − x3 + 2x4 + x6 = 3k … (9) x2 = x4 … (10) and − x3 + 2x6 = − 3k … (11) Now, subtracting equation (9) from equation (8) we get, x6 = 2k. Then from equation ( 11 ) we have, x3 = 7k. Also from equation (8) we have then, x4 = 4k , and from equation (10), we have, x2 = 4k. Thus we obtain: Now, x1 : x2 : x3 : x4 : x5 : x6 = 1 : 4 : 7 : 4 : 1 : 2. Hence the balanced form of the chemical equation is: Example 2: Let us now consider the balancing of the following chemical equation: Let the balanced equation be: ( x1 ) MgSO4 + ( x2 ) NaOH → ( x3 ) Mg(OH)2 + ( x4 ) Na2SO4 Equating the number of atoms of same kind of element on both sides of the above chemical equation we then obtain the following equations. For S: x1 = x4 … (13) For O: 4x1 + x2 = 2x3 + 4x4 … (14) For Na: x2 = 2x4 … (15) For H: x2 = 2x3 … (16) From equations (12), (13) and (15) we get: It can now be readily seen that both the equations (14) and (16) are satisfied with these values of x1, x2, x3, and x4. Now, x1 : x2 : x3 : x4 = 1 : 2 : 1 : 1. Hence, there is only one balanced form of the chemical equation under consideration and that is : MgSO4 + 2NaOH → Mg(OH)2 + Na2SO4 Example 3: Let us consider the balancing of the chemical equation: Cr2O72- + H+ + Fe2+ → Cr3+ + H2O + Fe3+ Let the balanced chemical equation be Since the number of atoms of each kind on both sides must be equal, we have then the following equations: For H: x2 = 2x5 … (18) For O: x1 = x5/7 … (19) For Cr: x4 = 2x1 … (20) Also, since the total charge before and after the reaction must remain the same, we must have, It is important to note that the equation (21) does not contain x5. This is only because of the fact that water (H2O) is neutral or zero-valent. Using equations (17) and (18), we have from equation ( 21 ), Now from equations (19) and (20) we get, Hence we have from equation (22), x6 = 6k. Also, from equations (17) and (18) we have then x3 = 6k, and x2 = 14k, respectively. Now, x1 : x2 : x3 : x4 : x5 : x6 = 1 : 14 : 6 : 2 : 7 : 6. Hence the balanced form of the chemical equation is: Example 4:Let us consider the balancing of the following chemical equation: Let the balanced form of the above chemical equation be Since the number of atoms of each kind on both sides must be equal, we have then the following equations: For H: x2 + 2x3 = 3x4 … (24) For N: x2 = x5 + x6 … (25) For O: 3x2 + x3 = 4x4 + x5 + 2x6 … (26) Now, let x1 = k1, so that from equation (23) we have, x4 = k1. Then equation (24) reduces to Also, equation (26) becomes Again let x2 = k2, so that from equation (27) we have x3 = ( 3k1 − k2 )/2. Equation (25) then becomes Also, equation (28) then becomes Now, solving for x5 and x6 we get from equations (29) and (30), x5 = ( 5k1 − k2 )/2, and x6 = ( 3k2 − 5k1 )/2. Thus we have x5 = ( 5k1 − k2 )/2, and x6 = ( 3k2 − 5k1 )/2. If k1 = 2, and k2 = 5, we have, x1 = 2, x2 = 5, x3 = 1/2, x4 = 2 , x5 = 5/2, and x6 = 5/2. Now, when reduced to simplest form we have, x1 : x2 : x3 : x4 : x5 : x6 = 4 : 10 : 1 : 4 : 5 : 5. Thus, the balanced form of the chemical equation under consideration is : This is the only balanced form that exists in the traditional literature. Again for a different choice such as k1 = 1, and k2 = 2, we have, x1 = 1, x2 = 2, x3 = 1/2, x4 = 1, x5 = 3/2, and x6 = 1/2. Now, when reduced to simplest form we have, x1 : x2 : x3 : x4 : x5 : x6 = 2 : 4 : 1 : 2 : 3 : 1. Thus, a second balanced form of the chemical equation under consideration is: Let us now proceed with the choice k1 = 5, and k2 = 13, so that we have, x1 = 5, x2 = 13, x3 = 1, x4 = 5, x5 = 6, and x6 = 7. Now, when reduced to simplest form we have, x1 : x2 : x3 : x4 : x5 : x6 = 5 : 13 : 1 : 5 : 6 : 7. Thus, a third balanced form of the chemical equation under consideration is: Proceeding in this way it is possible to obtain infinite number of independent balanced forms of the aforesaid chemical equation for all different choices of the values of k1 and k2. Example 5: Let us consider balancing of the following chemical equation: KMnO4 + H2SO4 + H2O2 → MnSO4 + K2SO4 + H2O + O2 Let the balanced form be ( x1 ) KMnO4 + ( x2 ) H2SO4 + ( x3 ) H2O2 → ( x4 ) MnSO4 + ( x5 ) K2SO4 + ( x6 ) H2O + ( x7 ) O2 Equating the number of atoms of each kind of element on both sides of the above chemical equation, we then obtain the following equations. For Mn: x1 = x4 … (32) For O: 4x1 + 4x2 + 2x3 = 4x4 + 4x5 + x6 + 2x7 … (33) For H: x2 + x3 = x6 … (34) For S: x2 = x4 + x5 … (35) Now let x1 = k1, so that from equations (31) and (32) we respectively have, x5 = k1/2, and x4 = k1. Then from equation (35), we also have, x2 = 3k1/2. Again let x3 = k2, so that from equation (34), we have, x6 = ( 3k1 + 2k2 )/2 and from equation (33), we have Thus we have x6 = ( 3k1 + 2k2 )/2, and x7 = ( 5k1 + 2k2)/4. As before, corresponding to a particular choice of the values of k1 and k2, it would be possible to get a particular balanced form of the relevant chemical equation. For example, if k1 = 2, and k2 = 5, we have, x1 = 2, x2 = 3, x3 = 5, x4 = 2, x5 = 1, x6 = 8, and x7 = 5. When reduced to simplest form we have, x1 : x2 : x3 : x4 : x5 : x6 : x7 = 2 : 3 : 5 : 2 : 1 : 8 : 5. Thus, the balanced form of the chemical equation under consideration is: This is the only balanced form of the chemical equation that exists in the traditional literature. Again if k1 = 2, and k2 = 1, then it can be readily seen that, we have in this case, Thus, the second balanced form of the above chemical equation is : 2KMnO4 + 3H2SO4 + H2O2 → 2MnSO4 + K2SO4 + 4H2O + 3O2 Now, if k1 = 2, and k2 = 3, then it can be readily seen that, we have in this case, Thus, the third balanced form of the above chemical equation is : In a similar manner, by choosing arbitrary pair of values for k1 and k2, it would be possible to generate infinite number of independent balanced forms of the chemical equation under consideration. However, where multiple options for a balanced equation are possible, it is important to recognize that equalizing the numbers of electrons transferred is determinative on the stoichiometric coefficients in the least complex final balancing. For this purpose the balancing of chemical equations by the “Oxidation number method” is now being considered below with reference to the chemical equations considered in Examples 1, 3, 4, and 5 above. For the first chemical equation, viz., NaNO3 + Zn + NaOH → Na2ZnO2 + NH3 + H2O, the skeleton equation that includes those reactants and products containing the elements that suffer a change in oxidation numbers is as follows:
Looking at this skeleton equation we find that the oxidation number of Zn increases by (+ 2 ̶ 0) or 2 and the oxidation number of N atom decreases by {(+ 5) ̶ ( ̶ 3 )} or 8. Now, in order that the increase and decrease of oxidation number are equal, there is a need of 4 atoms of Zn and 1 atom of N. Thus, the aforesaid chemical equation may be written as follows: It can now be readily seen that in order to get the salt Na2ZnO2 as a product, there is a final need of 7 molecules of NaOH. Thus, the balanced chemical equation is: For the third chemical equation, viz., Cr2O72- + H+ + Fe2+ → Cr3+ + H2O + Fe3+, the skeleton equation is as follows:
Looking at this skeleton equation we find that the oxidation number of Fe increases by {(+ 3) ̶ (+ 2)} or 1. Again the oxidation number of one Cr atom decreases by {(+6) ̶ (+3)} or 3. Hence, the total decrease in oxidation number of two Cr atoms = 2 × 3 = 6. Now, in order to equalize the increase and decrease of oxidation number, we are to put 2 in front of Cr3+, 6 in front of Fe2+ and 6 in front of Fe3+. Thus we obtain the following equation: Now, in order to ensure that the number of O atoms on both sides are equal, the above equation will have to be changed to the following form: The balanced chemical equation can then be obtained by equalizing the number of H atoms on both sides as follows: For the fourth chemical equation, Viz. P + HNO3 + H2O → H3PO4 + NO + NO2, the skeleton equation is as follows:
Looking at this skeleton equation we find that the gain in oxidation number of P is {(+ 5) ̶ 0} or 5. Again the loss in oxidation number of N for the conversion:
Thus, the net loss in oxidation number of N is (3 + 1) or 4.
Thus, the overall loss in oxidation number will then be (15 + 5) or 20, which is exactly equal to the total gain in oxidation number. Thus, the chemical equation under consideration can then be written as: The balanced chemical equation can then be obtained by equalizing the number of O atoms and N atoms on both sides as follows: For the fifth chemical equation, viz., KMnO4 + H2SO4 + H2O2 → MnSO4 + K2SO4 + H2O + O2, the skeleton equation is as follows:
Looking at this skeleton equation we find that the oxidation number of one oxygen atom increases by {0 ̶ ( ̶ 1)} or 1. Hence, the total increase in oxidation number for two oxygen atoms = 2 × 1 = 2. Again the oxidation number of one Mn atom decreases by {(+ 7) ̶ (+ 2)} or 5. Now, in order to take care of the fact that the increase and decrease in oxidation number are equal, there is a need to consider 2 atoms of Mn and 10 atoms of oxygen. Thus the aforesaid chemical equation may be written as follows: 2KMnO4 + H2SO4 + 5H2O2 → 2MnSO4 + K2SO4 + H2O + 5O2 Again, to get K2SO4 and MnSO4 as products, there is a need to consider 3 molecules of H2SO4. This ultimately leads to the balanced equation as: 2KMnO4 + 3H2SO4 + 5H2O2 → 2MnSO4 + K2SO4 + 8H2O + 5O2 This concludes the validity of the fact that equalizing the numbers of electrons transferred is determinative on the stoichiometric coefficients in the least complex final balancing. Conclusion The procedure for the algebraic method of balancing chemical equation offered in this paper is novel. At the same time it also reflects the efficiency of the algebraic method in regard to generating multiple balanced forms for some typical chemical equations. The application of this novel procedure for balancing chemical equations leads to a satisfactory answer to the fundamental fact that, the balanced form of a chemical equation is not always unique. It is definitely unique (with lowest possible integral coefficients) so long as {(m + n) – s} ≤ 1. In cases of chemical equations (not involving ions) with {(m + n) – s} > 1, and for those involving ions with {(m + n) – s} > 2, it is possible to generate infinite number of independent balanced forms of the chemical equation. In view of all of the above, the author notes that the novel idea presented in this paper is not only mathematically interesting, but that it deserves attention from the academic and pedagogical points of view to the relevant fields of science and engineering. References
|