|
Chemistry and Music have many connections in both practice and practitioners. Both have layered complexities underpinned by harmonics and mathematical roots [1]. Where music has its octave, chemistry has its octet. Famous in both fields, Alexander Borodin is known for composing “In the Steppes of Central Asia” and for a posthumous Tony award from his music used in a Broadway musical some two-thirds of a century after his death. He is also credited, along with Charles Wurtz [2], with independent co-discovery of the aldol condensation in 1872 while working with Emil Erlenmeyer. During Borodin’s time in Germany, he met Dmitri Mendeleev before the fellow Russian unveiled his “periodic system” in 1869 that would become the foundation of the periodic table.
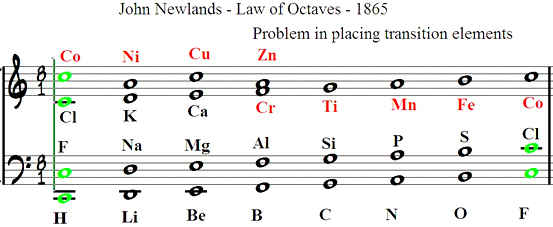
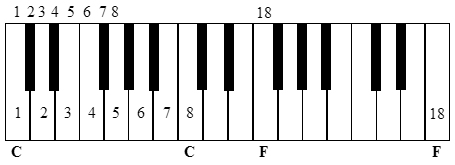
In 1865, English chemist John Newlands had divided 62 known elements into eight groups based on similar properties and published “On the Law of Octaves” [3]. As the inert gases were not known at this stage, it is easy, with hindsight, to see that every eighth element would have been in the same family up until the transition metals. This pitfall with transition metals (Fig. 1) meant Newlands’ initial findings were dismissed by the Society of Chemists (forerunner to the RSC) but he was eventually recognized with the Davy Medal in 1887. Yet, there is in fact a connection between piano scales and the transition metals that may be useful in teaching students the commonalities of the periodic table. An octave is only seven steps as the first and eighth notes are repeated: if middle C is note 1, the 8th note is also C. However, beyond the eight ivory notes of the C major scale, there are five ebony notes that lay untouched. These represent unfilled d orbitals, if you will. Again, starting from middle C as note 1, the 18th note in the C major scale is an F. However, starting again from middle C but playing every note (C, C#, D, D#, and so on), the 18th note is also an F but one octave lower (Fig. 2). This also works starting from A, B, D, E or G (but not F).
Thus, the initial failing of the Law of Octaves to incorporate transition metals has a fairly simple musical solution. Using either an octave or the aforementioned “octadecave” (Fig. 2) may be useful in promoting understanding of the periodic table, not only in introductory chemistry but to the general public, as well. If an actual piano is available, it is ideal as the very sound of the note itself is generated by a combination of first row and transition elements: high carbon steel piano wire. References
Note: The author’s father, Prof. Oliver C. Houston, Jr., taught organ and music theory at Graceland University in Lamoni, Iowa, for over 48 years. This article is dedicated to his memory. |