|
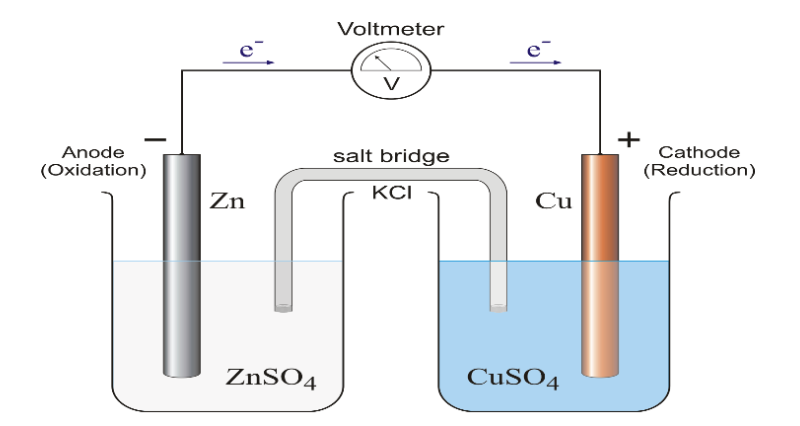
Introduction Environmental issues are considered one of the most important problems that human-kind faces in the modern era. Its types rang the bell of the waist around what must be done to effectively reduce the risks facing the earth. International, regional, and local conferences have been held to reduce environmental problems. A wareness has increased on environmental issues and countries have begun to try to reduce pollutants and produce environmentally friendly and biodegradable materials. Interest began to increase until the individual, in his school, his home, and his community, became morally obligated to be behaviorally friendly to the environment. Environmental issues have been raised in the curricula of schools since the first grade, in order to instill awareness among students of this issue that has become life threatening. Scientists have begun, in each field, to develop methods and materials aimed at reducing the production of environmental pollutants and even reaching what is known as preventing pollution, if possible. In the field of chemistry, what is known as narrow-field chemistry has emerged at the end of the last decade under the auspices of the United Nations Culture and Science Organization (UNESCO). Educational workshops in many countries have developed a new mechanism for the implementation of laboratory experiments in schools mainly aimed at reducing the amount of pollutants as much as possible. This indicates that a revolution in chemistry education has begun. Experimental work is an integral part of science education and this is what you can get when you ask a teacher or science teacher. The experimental work should include active participating students. Subsequently, chemistry is primarily an experimental topic ... and education in chemistry must contain an unavoidable experimental component [1]. Nevertheless, the truth in science education is completely different, and this can be identified by asking any honest and sincere science teacher, and the frightening truth will always be discovered. In most science rooms in schools there are no practical activities. In rich societies you may find practical alternatives; but in poor societies you will find everything written on the board only. Likewise, the experimental work in these societies is old, retroactive, eroding and extended until students graduate from school with simple practical experience and a modest understanding of science [1]. Hence, this problem has been identified for several years through UNESCO as well as IUPAC-CTC (International Union of Chemistry and Theoretical Chemistry and Applied Chemistry - Center for Chemistry Education). A number of founders have taken it upon themselves to start the International Narrow Band Chemistry Program, which started in 1996 and aimed to tackle problems by developing a small-scale, low-cost method. This is due to the widespread belief that experimental work is an essential and necessary part of teaching chemistry [1]. There are several definitions for the small-scale chemistry concept, including : "Small-scale chemistry is simply the process of performing and conducting chemical experiments within a greatly narrowed range" [2]. It is also a way to teach traditional laboratory chemical experiments in an exciting and attractive way to integrate with modern analysis techniques and implement them, even in the classroom, as well as to improve and strengthen educational curricula and raise scientific standards, which is a method for teaching chemistry by doing chemistry [3]. It is also a low-cost, safe and time-saving innovation based on keeping educational chemistry models. It is also not a curriculum, but it is a very flexible method for teaching chemistry at all levels by using inexpensive materials and common plastic materials, such as lollipops, plastic cups, disposable pipettes, petri dishes and syringes. And, so that there is no misconception, the narrow-band chemistry describes body or behavior, more than describing size [4]. This concept has been applied in many countries of the world, especially the United States of America, to include schools and the first stage of study in universities. There are many educational and environmental goals that can be achieved by applying this concept at all educational levels. Our interest in our environment, the development of the educational process in Jordanian schools, and participation in the research and development of experiments carried out within the activities established in curricula, lead us to choose the topic of galvanic cells (which is one of the main topics in the chemistry curriculum scheduled for 9-12 grade students) and this subject, whose experiences are rarely implemented due to students᾽ lack of sufficient time and materials to go to the laboratory to do the experiment. So, it was necessary to review and develop these experiments so that we get to know the ways of implementing them in a new, environmentally friendly and low-cost scientific method. The galvanic cell’s modification to be more environmentally friendly, inexpensive and simple has been reported previously [5-7]. The most important objectives of this research are: 1) Developing a significantly lower cost small-scale Zn-Cu galvanic cell instead of the traditional one that is cost prohibitive and cannot be carried out in a class room (Figure 1).
2) Study the effect of volume reduction on the amount of potential difference generated compared to the global standard value. 3) Study the effect of low concentration in this developed cell compared to the standard potential value of Zn- Cu galvanic cells. 4) Identify the oxidation and reduction reactions that occur on the electrodes. 5) Calculating the costs of implementing the experiment compared with the cost of the standard Zn- Cu galvanic cell. 6) Calculating that amount of solutions that were not consumed and not disposed of in the environment. Experimental Preparation of electrolytes and electrodes A 1 M of ZnSO4.7H2O and 1 M of CuSO4.5H2O were prepared.Copper sulfate and z inc sulfate solutions with the concentrations of 0.1, 0.01, 0.001 M were prepared by serial dilution for the standard zinc sulfate and copper sulfate with distilled water. Preparation of galvanic cells To build the small galvanic cell (Figure 2), Zn| ZnSO4(1.00M)||CuSO4(1.00M)|Cu, 5 mL of 1 M zinc sulfate was put in a small vial and another 5 mL of 1 M copper sulfate was put in another small vial; then, the two vials were connected by saturated filter paper which was soaked in sodium chloride solution for 5 minutes. Finally, the galvanized nail was inserted in zinc sulfate solution where a copper wire was inserted in copper sulfate solution. These electrodes were then connected with the multimeter to measure the generated potential difference. The readings were recorded and carried out in triplicate.
To build a rolled small-scale galvanic cell, Zn| ZnSO4(1.0M)||CuSO4(1.0M)|Cu (Figure 3), 1 mL of 1 M zinc sulfate was put in a small dish and another 1 mL of 1 M copper sulfate was put in another dish, then a filter paper was soaked in this solution for 5 minutes .The galvanized nail was rolled by the filter paper which was soaked in zinc sulfate solution; afterward, this part of the cell was rolled by semi-permeable paper and rolled again by the filter paper which was soaked in copper sulfate. Finally, all these parts were rolled with 10 rolls of copper wire and connected with the multimeter from the copper wire and galvanized nail to measure the generated potential difference. The readings were then recorded, and the experiment was carried out in triplicate.
To prepare the other galvanic cell the same procedure for the small-scale galvanic cell was carried out with the concentrations of the solutions changed in accordance to the experiment. The galvanic cells were prepared, and the generated potential differences were examined. Each experiment was carried out in triplicate. To investigate the effect of concentration on the generated potential difference in the rolled small-scale galvanic cell, the following galvanic cells were prepared and the generated potential differences were recorded. Each experiment was carried out in triplicate:
Parameters affecting the generated cell potential were investigated using the separated cells. At first, the effect of reducing the solution's volume on the generated potential was investigated (Figure 4). Regarding the small-scale galvanic cell (Figure 2) where 5 mL of each solution was used, the average of the generated potential of 1.045, 1.043, and 1.035 volt was 1.041±0.0043, which is 94.6% of the potential of the Zn-Cu galvanic cell (1.10 volt) at the standard conditions. Also, the effect of size and volume of the rolled small-scale galvanic cell was recorded. The obtained potentials for the rolled small-scale galvanic cell(Zn|ZnSO4(1.00M)||CuSO4(1.00M)|Cu were 1.015, 1.036, and 1.033 volt with average of 1.028±0.0093, which is 93.0% of generated potential of Zn-Cu at the standard conditions.
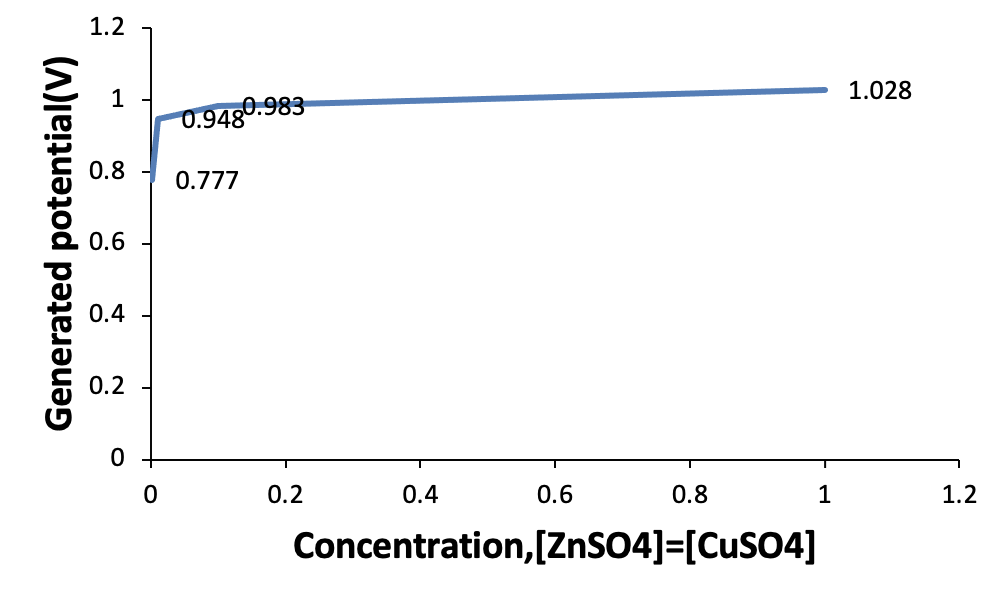
The effect of zinc sulfate and copper sulfate concentration on the generated potential was examined. Figure 5 shows that as the concentration of zinc sulfate and copper sulfate decreases the obtained potential decreases.
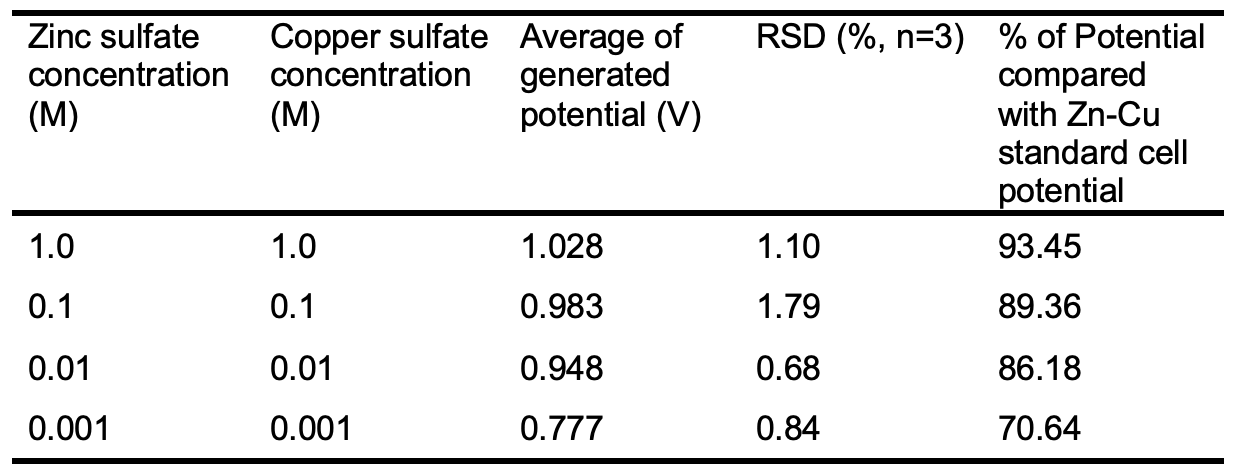
Table 1 shows the results of changing the concentration of zinc sulfate and copper sulfate on obtained potentials.
As Table 2 shows, the generated potential difference of the Zn-Cu galvanic cell decreases as the concentration of both solutions ([Zn2+] =[Cu2+]) is diluted from 1 M to 0.001 M, even though the generated potential for the more diluted solutions ([Zn2+] =[Cu2+] =0.001M) is 70.64% of the standard potential of the Zn-Cu galvanic cell.
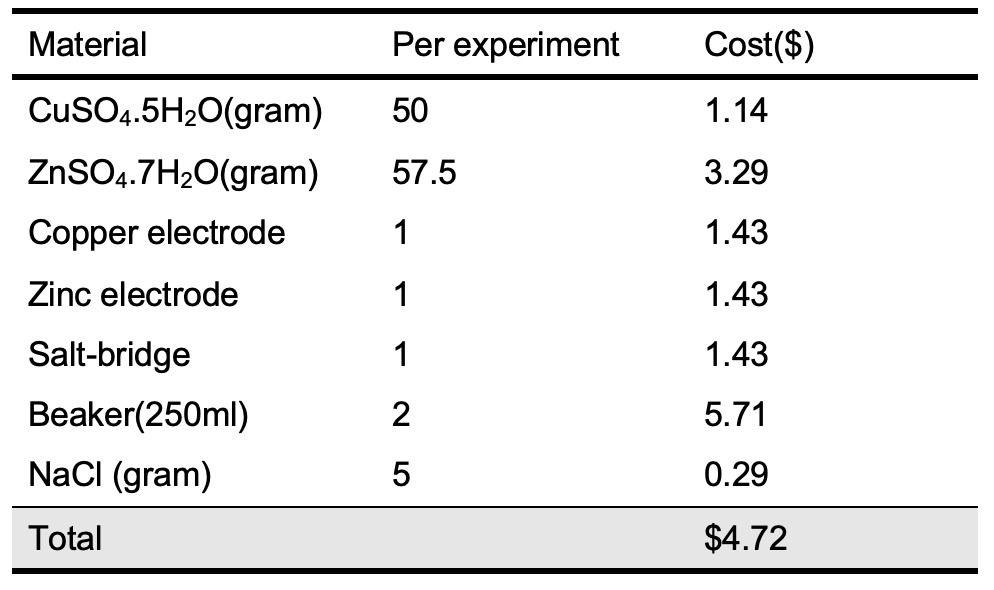
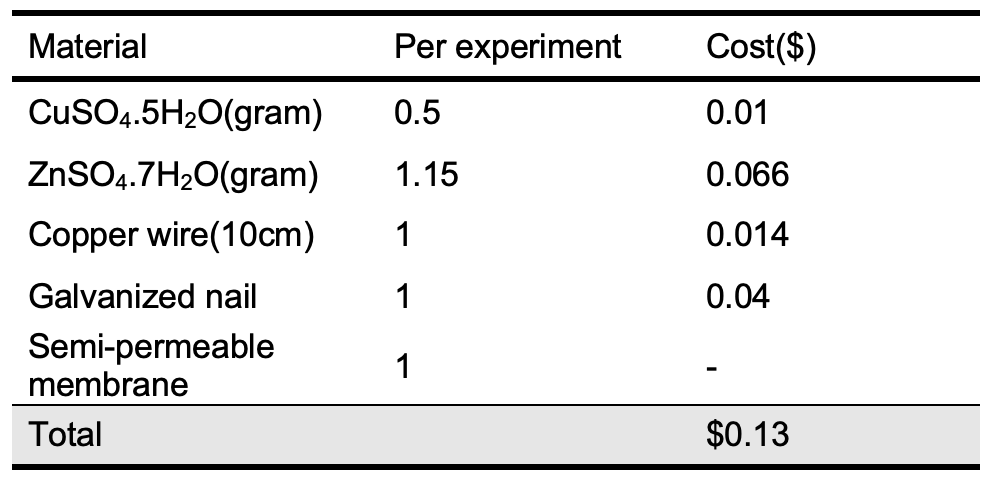
The cost of the standard Zn-Cu galvanic cell materials compared with the cost of the rolled small-scale model was carried out as shown in Tables 3 and 4 according to Jordanian local market prices for chemicals and materials.
According to the data mentioned in Tables 3 and 4, the cost of one rolled small-scale Zn-Cu galvanic cell is 0.88% of the cost of one of the standard Zn-Cu galvanic cells. This is considered an excellent indicator for the applicability of this cell in the labs and classrooms individually or within groups. Also, there is no disposal solutions where 1 mL of each solution was used via soaking the filter papers during cell preparation. Conclusion A simple and cheap small-scale Zn-Cu galvanic cell with zero waste was successfully constructed and used as a teaching material in electrochemistry topics in teaching laboratories and class rooms. Based on this design the students will be able to construct a small- scale battery able to operate low resistance circuits and design more small-scale electrochemical cell. Acknowledgments The author would like to thank the Department of Chemistry at Al-Balqa University for providing laboratory facilities and support.References
|
||||||||||||||||||||||||||||||||||||