|
Introduction Diabetic polyneuropathy “DPN” is among the most common causes of chronic problems of type-2 diabetes, affecting upwards of half of those with the disease. [1]. In Basrah, southern Iraq, a study reported that the main risk factors for foot ulcers are peripheral neuropathy, impaired wound healing process, decreased defense mechanisms, and impaired immunity, which can result in amputation and death. The large majority of Iraqi type 2 diabetes patients who have more extended periods of DM and hyperglycemia have a greater possibility of ending with diabetic foot ulcer outcomes [2]. DPN symptoms include numbness and tingling pain, weakness, loss of senses, and ataxia. [3,4]. Aside from the significant morbidity, mortality, and decreased quality of life experienced by patients, DPN is regarded as the most important initiating health risk for diabetic foot ulcers and non-traumatic lower leg surgical removal [5-7]. Diabetic nerve damage is caused primarily by elevated glucose levels in the blood (hyperglycemia), which disturbs the capacity of nerve cells to interact, resulting in a weakness of the bloodstream providing nutrients and oxygen to the nervous system [8,9]. Furthermore, axonal vitality is lost as a result. Thus, inflammatory cytokines can undoubtedly cause nervous dysfunction as well as, ultimately, death of cells [10]. Other risk factors include body mass index (BMI), smoking, diabetes period (time span), age, hypertension, fasting glucose, and HbA1c levels [11-16]. Diabetic peripheral neuropathy (DPN) is one of the most prevalent and disabling complications of diabetes mellitus (DM), which occurs in more than half of affected individuals. Some previous studies reported that more than 25% patients with T2DM may develop DPN, although up to half of them may remain asymptomatic. Clinically, the diagnosis of DPN often depends on the presence of patient-reported symptoms and physical signs, thus delaying the detection of subclinical diabetic peripheral neuropathy (sDPN). Therefore, identifying sDPN at an early stage and detecting the predisposing factors for its development may slow, stop, or even reverse the progression of DPN and avoid the occurrence of DPN-related morbidity and complications (Risk Factors for Subclinical Diabetic Peripheral [1] Neuropathy in Type 2 Diabetes Mellitus). Aldose reductase is indeed a cytosolic NADPH-dependent oxidoreductase, which stimulates the decrease of aldehydes and carbonyls, including monosaccharides, with the primary function of converting glucose to sorbitol [17]. It is found in non-uniform levels in most mammals with high levels in the eye (retina, cornea, and lens), peripheral nerves, kidney, and myelin sheath tissues, which are frequently involved in diabetes-related complications [18]. The development and progression of diabetic neuropathy are thought to be influenced by four distinct pathways. These pathways are named intracellular production of advanced glycation end products, “AGE” precursors, hexosamine pathway, protein kinase C “PKC” activation, and polyol pathway, and they depict metabolic abnormalities in nerve physiology. High glucose levels activate the polyol paths (the enzyme aldose reductase converts glucose to sorbitol). Aldose reductase consumes NADPH when glucose is converted to sorbitol, reducing glutathione production and increasing susceptibility to intracellular oxidative stress. The increased activity of the polyol pathway lessens myo-inositol, which leads to peripheral neuropathy [19-22]. According to the current research, hyperglycemia could perform a crucial role in the pathophysiology of complications via various mechanisms such as aldose reductase (AR) related polyol path, increased formation of advanced glycation end products, and excessive oxidation stress.
Materials and Methods
Subjects The study includes 81 subjects: adult males ages ranging from 33-78 years, twenty-seven (27) healthy subjects as a control, and 54 patients with diabetic type 2. The patient population was divided into 2 groups: diabetic type 2 group (27 T2DM) and Diabetic peripheral neuropathy group (27 DPN). The study was conducted at Mustansiriyah University College of Science, Chemistry Department from 10 October 2021 to 20 December 2021. All patients and the control were collected from Baghdad, Iraq, Al Imamian, Al Kadhmiyain, Medical City. Both the patients and the control were evaluated using biochemical tests, and immunological and biomedical laboratory tests. Exclusion criteria: all subjects were without any noticeable inflammatory anomalies, tumors, or long-term illnesses. Patients' baseline characteristics and control data were collected from patient health registers. The following information was gathered: age, sex, DM duration, smoking status, family history, and hypertension. The identification of DPN was confirmed by previous medical reports from the Specialized Endocrine and Diabetes Center of Iraqi Ministry of Health, and positive results were obtained from neurologic medical tests and peripheral nerve tests. In addition, a medical evaluation involving a review of each patient and clinical signs and symptoms of psychological examination was recorded (tingling pins and needles or numbness in the hands and feet; sharp, burning severe pain, especially at night; loss sensitivity to touch; muscle weakness).
Sample Collection Four milliliters of blood were taken from fasting patients, and the control group's blood samples were obtained during the hours of 9:00 am to 12:00 pm. Each blood sample was divided into two parts. The first one: 2 milliliters of blood was transferred into EDTA-containing tubes for HbA1C measurements. Second, 2 milliliters were transmitted into gel tubes for biochemistry measurements (glucose and aldose reductase). For 30 minutes after that, the serum was centrifuged for 10 min at 3000 rpm, divided into Eppendorf, and kept at -20oC until the related parameters were measured.
Sample Analysis The body-mass-index (BMI) of the subjects separated the research-measured data into normal (19≤ 24.9) and overweight (25≤ 29.9). Using the formula: BMI= Weight (kg) / Height (m2) [24]. Additionally, fasting glucose level and enzymatic hemoglobin A1C (HbA1c) were calculated utilizing an automated clinical chemistry analyzer (Atellica-CH930-Siemens, German), while aldose reductase was calculated by sandwich ELISA method using (AR ELISA Kit MyBioSourse\USA).
Statistical Analysis The statistical impacts of the study were estimated by The SPSS version 26 ANOVA statistical software to calculate the quantitative variables for parameters reported in the research. It comprised the mean, standard deviation (SD), and p-values (ANOVA) and using an analysis of variance, Pearson's correlation is calculated. Conducted to determine significant differences in different variables among all groups, data analysis significance was found at p-value <0.05 and extremely significant at (p-value <0.01).
Results and Discussion The findings in Table 1 describe the age, hypertension, BMI of patients and healthy subjects, which shows the range for all study groups was between 35-82 years for the three groups. The mean ± SD of their ages were: 53.450±8.0126 and 55.125±10.754 and 50.250±10.460 for the three groups, respectively, with a significant p-value (p>0.05). The mean hypertension (mmHg) with a standard deviation (SD) of control individuals is 1.851±0.3620 ±7.441, while the mean ± SD of hypertension (mmHg) of T2DM patients is 1.555±0.506. Additionally, the mean±SD for DPN patients was 1.407±0.500 with a significant p-value (p>0.01). The BMI mean values for patients and control groups were found to be approximately similar with a non-significant p-value (p>0.05).
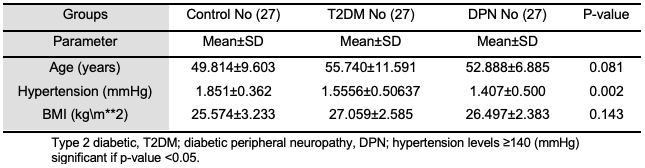
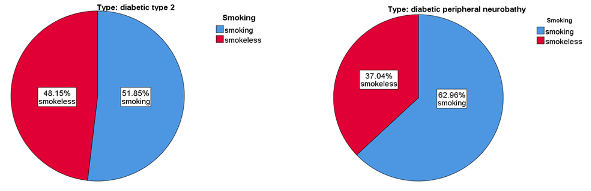
Figure 1 shows that most of this study's participants were smokers, 62.96% and 51.85% for DPN and T2DM, respectively. Furthermore, the family history of disease results were 74.07% and 51.58% for DPN and T2DM, respectively [25]. Identification of independent risk factors for diabetic neuropathy progression in patients with type 2 diabetes mellitus. Journal of International Medical Research who had a family history of disease. Other research reported that in every category studied, sick people were much more likely to have a significant history of diabetes and complications such as “DPN”. The family history of diabetes and major complications were associated with peripheral neuropathy [26,27].
In addition, Figure 2 shows that most of the participants in the study were smokers (62% and 51% for DPN and T2DM, respectively). Furthermore, most of them have a family history of the disease for DPN and T2DM (74% AND 51% for DPN and T2DM, respectively). These results agree with (28-30) have a family history of the disease. Other research reported that in every category studied, sick people were much more likely to have a significant history of diabetes and complications such as “DPN”. Family history was linked with peripheral neuropathy patients [31,32].
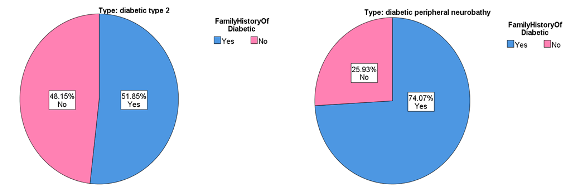
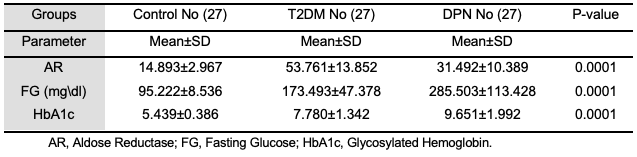
Also, the comparison in duration of disease in Figure 3 showed highly significant differences for the DPN group. It was over a longer period compared to the T2DM group (p<0.05) [14]. According to the study's findings, there is also a highly significant increase in the level of hypertension in the DPN group, especially compared to the T2DM and control groups (p<0.05). It agrees with previous studies that hypertension and diabetes are independent risk factors and have significant additive interactions with disease [15]. Table 2 illustrates the clinical parameter analysis for the three DPN patients with T2DM and control groups. Analyzing the current data of participants (n=81) showed that the variance of aldose reductase in the analysis was significant. The mean of aldose reductase for T2DM was noticeably higher than for both DPN and control groups (p>0.05). Many other studies showed that the relationship between aldose reductase (AR) and hyperglycemia would be cause the development of diabetic type 2 to diabetic peripheral neuropathy. AR levels were significantly increased in T2DM patients, and the accumulation of polyol pathways was mediated by AR and intermediate glycation products or advanced glycation end products (AGE), which induced oxidative stress. Also, modified protein kinase C activities are considered to be responsible for diabetic peripheral development [34,35]. The mean value of fasting glucose level for the DPN group was significantly higher than that of T2DM and control groups (p< 0.05), which is similar to other previous studies [16]. The comparison of the HbA1c between the groups showed a significant increase for the DPN group compared to T2DM and control groups. It agrees with other studies that reported that the difference between DPN groups compared to T2DM and control groups was statistically significant (p<0.05) [16,33].
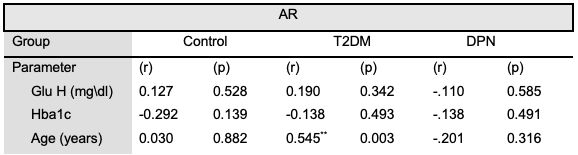
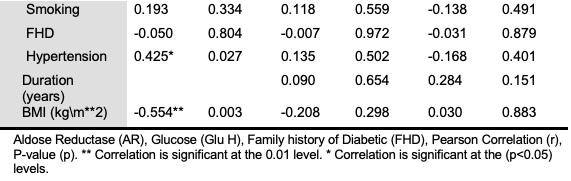
The results in Table 3 with Figure 4 show that the AR had a strong positive correlation with Age (years) (r= 0.545**) (p= 0.003) in the T2DM group, however, with a moderate “positive correlation” with hypertension (r= 0.425*) (p= 0.027) and a strong “negative correlation” with BMI in control group (r = -0.554**) (p= 0.003).
In this current study, there was also no relationship between the alteration in HbA1c and glucose with alteration in the level of aldose reductase. It corresponded with other studies that reported no statistically significant correlation observed between the level of AR and the average of HbA1c and fasting blood glucose [36,37]. Although there's reasoned evidence that points to the AR as a biologically probable crucial gene for developing type 2 diabetes complication diseases [38], so much research has found that the AR is linked to diabetic retinopathy, neuropathy, and nephropathy, possibly due to an involvement of the polyol pathway in the pathophysiology of naval vessel diabetic complications [39-41]. Furthermore, the alteration in AR levels in the DPN group are an obvious characteristic that control group. A future study is needed to fully understand the relationship between the serum AR and DPN. Because other studies indicated that there is a high incidence of AR in T2DM patients and is linked to diabetic peripheral neuropathy. Quite a lot of pathological modifications in nerve cells, nerve fibers, and vascular endothelial cells can result in nerve dysfunction and, eventually, death. It has been suggested that nerve damage might have developed during the pre-diabetes stage. Post-load hyper glucose levels may be the primary mechanism causing increased oxidative stress, vascular dysfunction, and stimulation both for the polyol pathway and the protein kinase C, resulting in impaired nerve cell metabolism and damage to DNA [42].
Conclusion The increase in the mean value of aldose reductase was significant (p<0.05) for the DPN group compared to others. The mean value of glucose HbA1c for DPN participants was considerably higher than those in the T2DM and healthy controls groups (p<0.05). It can be concluded that the AR level was an important parameter for following the T2DM cases complicated by DPN. This is because the enzyme converts glucose to sorbitol, which can accumulate in cells, leading to oxidative damage. By monitoring AR levels or its activity, clinicians can potentially gain insights into the progression of diabetic complications. Inhibition of aldose reductase is being explored as a therapeutic strategy to prevent or delay these complications.
References
|
|||||||||||||||||||||||||||||||