|
Introduction Juniperus is one of the major generals in the Cupressaceae family, it includes about 50-75 species distributed throughout the world, depending on the taxonomic classification [1], and it has more than 220 varieties [2]. Junipers belong to the Pinophyta (Conifers) division of plants, producing many biologically active metabolites, which contributes to their various biological activities [3]. Juniperus excelsa is an evergreen tree with a narrow, pyramidal crown when young, becoming more spreading with age; it can grow 4 - 12 meters tall, sometimes reaching 20-25 meters. The bole on more giant trees can reach 150-250 cm in diameter [4]. It grows on dry rocky slopes in hills and mountains at an altitude of 150-2700 m [5]. Juniperus excelsa, commonly called the Greek juniper or Grecian juniper, is a juniper found throughout the eastern Mediterranean, from northeastern Greece and southern Bulgaria across Turkey to Syria and Lebanon, Jordan, the Caucasus mountains, and southern coast of Crimea [6]. This species of Juniperus excelsa has been divided into 2 subspecies (subspecies: M.Bieb and subsp. polycarpus) by vargon: one with a distribution in southeastern Europe, the Crimea, southern Turkey mainly to Lebanon; the other, a continental component extending from northern Turkey to Kyrgyzstan and Pakistan [7]. The Juniperus excelsa M.Bieb strain is the subject of this paper, grows in temperate regions throughout the eastern Mediterranean mountains and is widespread in Lebanon. In Syria, it exists in the Qalamoun mountains and in the Latakia mountains. It is locally known as “lezzab” or “chajarit al bakhour” [8]. Juniperus excelsa was formerly treated in a looser sense to include the species Juniperus polycarpos and Juniperus seravschanica. The three are now generally seen as distinct, though they are all similar and have similar properties. It can be challenging to separate out the various uses attributed originally to Juniperus excelsa to any of these three species and, certainly on the traditional level, they are very likely to apply to all three. All the medicinal uses we know of are listed here (where possible, we point out if they were specifically attributed to what is now one of the three species) [9]. The studies on the Syrian juniper tree (Juniperus excelsa) found in the Qalamoun region are very limited and almost unavailable, so this work aims to determine the chemical composition of essential oils contained in the leaves of Juniperus excelsa to determine the antioxidant activity. Figure 1 shows Juniperus excelsa M.Bieb trees.
Materials and Methods The preparation of solutions and the conduct of chemical analyses were carried out at the laboratories of the Private Syrian University. The analysis of antimicrobial activity was carried out at the National Commission for Biotechnology in Damascus, while the analysis of essential oil by gas chromatography was carried out at the Atomic Energy Commission in the Syrian Arab Republic. Collection and Preparation of Plant Leaves The plant, Juniperus excelsa M.Bieb, was collected precisely on 14 July 2023 from the Al-Khushaa region in the Al-Qalamoun mountains, Ras al-Ma'arra village, Yabroud area, Damascus countryside, Syria. The fresh leaves and green fruits were separated from the twigs and washed with tap water, then the drying process was carried out for 24 hours in a dark and dry place at room temperature.
The grinding process was carried out with an electric grinder immediately before work until a grain size of less than or equal to 0.2 mm diameter was reached. Figure 2 shows Juniperus excelsa M.Bieb leaves and branches bearing berry fruits. Preparation of the Essential Oil The steam distillation method was employed using a Clevenger-type apparatus to extract the essential oil. The process was conducted in two repetitions. Each time, 100 grams of the ground leaves or fruits were subjected to steam distillation for 4 hours, along with 1000 milliliters of distilled water [17]. DPPH Radical Scavenging Assay The evaluation of Juniperus excelsa essential oil's (EO) antioxidant properties was conducted through the DPPH Radical Scavenging Assay (RSA). To initiate the assessment, a stock solution of DPPH was prepared by dissolving 4 milligrams of DPPH in 100 mL of 99% ethanol. Filtration of the DPPH stock solution with ethanol yielded a usable solution with an absorbance of approximately 0.886 at 517 nm. Subsequently, 1 mL of the workable DPPH solution was mixed with 50 µL of EO in a test tube. The tubes were then placed in complete darkness for a duration of 30 minutes, after which the absorbance at 517 nm was measured. The following formula was employed to calculate the percentage of antioxidants or Radical Scavenging Activity (RSA) [18]:where: Ac—Control reaction absorbance; As—Testing specimen absorbance. Gas chromatography-mass spectrometry Essential oils of leaves and fruits of Juniperus excelsa were analyzed by gas chromatography with electron-ionization mass-selective detector, according to the previously published procedure [19]. In brief, 1 μL of the sample dissolved in hexane (1;10) was injected into a split/split-less inlet at 240°C, with a split ratio of 1:50. Helium (purity 99.999%) was used as a carrier, with a constant flow of 1 mL/min. The separation was achieved on the HP-5MS 5% Phenyl Methyl Silox column (325°C: 30 m x 250 µm x 0.25 µm) using the following temperature program: start at 40°C, 2°C/min , then 2.5°C/min to 70°C, then 2°C/min to 130°C , then 2.5°C/min to 160°C , then 5°C/min to 200°C , then 10°C/min to 240°C, and hold for 10 min. The eluate was delivered to the mass spectrometer via a transfer line held at 260°C. The ion source temperature was 240°C, electron energy was 70 eV, and the quadrupole temperature was 150°C. Data was acquired in scan mode (m/z range 42–850). The compounds were identified by comparison of mass spectral with data libraries (Wiley Registry of Mass Spectral Data, 7th ed. and NIST/EPA/NIH Mass Spectral Library 05). Relative amounts of components, expressed in percentages, were calculated by normalization measurement according to the peak area in total ion chromatogram.
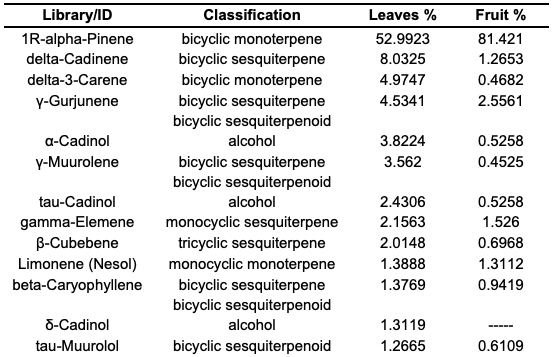
Results and Discussion The resulting essential oil exhibited a very pleasant and strong aroma, while its color appeared transparent without any obvious color in the case of the leaves and fruits. The average percentage of essential oil (extracted by Clevenger type) relative to the raw material of the leaves was 1.38%, while the essential oil yield of the fruits was 0.8%. This study worked in the identification of antioxidants within the essential oil of Juniper excelsa leaves and fruits by evaluating 2,2-diphenyl-1-becquerylhydrazyl (DPPH) radical scavenging activity. DPPH screening is a well-established, cost-effective and effective methodology for measuring antioxidant capabilities. It is based on the use of free radicals to assess the ability of substances, to act either as hydrogen donors or scavengers of free radicals. This technique involves the reduction of a DPPH, which is a stable free radical, and interaction with an unpaired electron leads to intensive absorption at 517 nm, which appears as a purple color. For example, the reactivity of juniper essential oil has been demonstrated by the ability of oils to act as hydrogen atoms or electron donors in the transformation of stable purple to reduced yellow. A free radical scavenging (FRS) antioxidant, for example, reacts to DPPH to form DPPH-H, which has a lower absorbance than DPPH because of the lower amount of hydrogen. It is radical in comparison to the DPPH-H form because it causes decolorization, or a yellow hue, as the number of electrons absorbed increases. Decolorization affects the lowering capacity significantly. As soon as the DPPH solutions are combined with the hydrogen atom source, the lower state of diphenylpicrylhydrazine is formed, shedding its violet color [20]. After the mathematical relationship of the antioxidant activity was applied to the essential oils that were extracted from leaves and fruit of the Clevinger-type (antioxidant activity= [(Ac−As)÷Ac]×100), it was showed (55% – 38%), respectively. The results can be interpreted by comparison with data from previously published articles as reference [21]; which indicated that the main compounds of oils showing high antioxidant activity were α-pinene (33.7%) in juniper berry, limonene (74.6%) in celery seed, benzyl acetate (22.9%) in jasmine, myristicin (44%) in parsley seed, patchouli alcohol (28.8%) in patchouli, citronellol (34.2%) in rose, and germacrene (19.1%) in ylang-ylang. In the same paper [21], it was noted that the scavenging abilities of various bear root essential oils ranged from 39% – 90% (39 for Angelica seed oil to 90 for jasmine). The chemical composition of Juniperus excelsa leaves and fruit essential oil determined by Gas chromatography-mass spectrometry (GC–MS) GC/MS is presented in Table 1. Twenty-six components for leaves and 21 for fruits, representing total detected constituents, were identified: 100% for leaves and 98.11% for fruits. The terpenes percentages were: monoterpenes (3.84% – 4.76%), and cyclic monoterpenes (23.07% – 28.57%), cyclic sesquiterpenes (46.15% – 57.14%), cyclic sesquiterpenoids (26.94% – 9.53%) of leaves and fruits, respectively.
The main constituents of the essential leaves and fruits oils of J. excelsa were α-pinene (52.99% – 81.42%), delta-Cadinene (8% – 1.26%), dl-Limonene (1.38% – 1.311%), gamma-Elemene (2.15% – 1.53%), delta-3-careen (4.97% – 0.43%), gamma-Gujunene (4.53% – 2.55%), and α-Cadinol (3.82% – 0.525), respectively. Figure 3 shows the chemical formulas of the main compounds.
The results of this study did not completely agree with the published data of previous studies. At the same time, the compound α-pinene is consistent with most studies [8,21,22], as it constitutes the most abundant compound in the essential oil. In contrast, some studies, which are very few, indicated that sabonene was the most abundant [23], while in other studies, limonene had the highest percentage [24], the largest in existence. A comparison of the numbers and quantities of components found in the essential oils of these plants grown in different parts of the world indicates that the oil composition of individual plants may vary significantly due to climate, growing region, time of collection, etc. These differences are very common.
Conclusion In previous studies, researchers explored the different natural products that plants make. They focused on terpenes, a significant group of substances in conifer trees. Terpenes help protect plants from harmful invaders like pathogens and plant-eating animals. Some terpenes also work as antioxidants, which can reduce damage from stress. Terpenes are affected by various stresses like lack of water, changing temperatures, pollution, and attacks from pathogens. However, how they react to stress depends on the type and strength of the stress. Each conifer species has its typical terpene mix, but even trees of the same kind can have different terpene patterns.
Recommendations Alpha and gamma terpenes found in extracts of this plant have been reported to have high antioxidant properties. In addition, some studies have shown that the monoterpene components of juniper essential oil enhance resistance to oxidative stress of organisms, and the essential oil is also strongly antifungal where the main compound responsible for these antifungal activities is delta-3-carene. Therefore, we recommend studying this type of Syrian juniper as an antifungal and antibacterial.
Author Contributions Investigation, project administration, supervision, and software: D. Mariam. Writing–review & editing, formal analysis, and methodology: A. H. Manar. All authors have read and agreed to the published version of the manuscript.
Funding: This research received no external funding.
Data Availability Statement: Data is contained within the article.
Conflicts of Interest: The authors declare no conflict of interest. References
|
||||||||||||||||||||||||||||