|
Introduction Onion (Allium cepa L) is a worldwide food resource due to its nutritional and medicinal value. World onion production has increased by at least 25% over the past 10 years with current production being around 44 million tonnes annually, making it the second most important horticultural crop after tomatoes. According to the Food and Agriculture Organization of the United Nations, in 2021, the global production of onion was 107 million tons [1]. Increasing demands and production of onions have begun generating increased amounts of onion byproducts during processing, such as peel, and their disposal poses a burden on the environment. The processing of the onion generates a vast amount of onion peel or skin that is thrown away as a waste. Dry onion peel is rich in bioactive compounds such as polyphenols, flavonoids, tannins, and other secondary metabolites, especially free quercetin. The composition of onion peel changes based on different varieties, agronomic conditions of the region in which they were cultivated, and the extraction techniques used [2-3]. Quercetin (2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-1-benzopyran-4-one), found abundantly in onion peel and containing more than 77-fold that found in the edible part of the onion, is one of the major plant-derived bioflavonoids. This compound mainly exists in dry onion peel in aglycone form (67-86% of the total quercetin), with only a small proportion of quercetin appearing in the glycoside form [4]. This natural compound is interesting due to its wide range of bioactive activities and shows not only antioxidant properties like all the bioflavonoids but also anti-inflammatory, antiviral, antibacterial, anticarcinogenic, hepatoprotective, cardiovascular and anti-platelet effects. It has been known to have anti-obesity, antidiabetic, and antihypertensive effects in animal and human studies as well. Quercetin could also sensitize resistant cancer cells to chemotherapy and synergize the effects of drugs on nonresistant cancer cells [5-13]. Due to the antioxidant characteristics of onion peel, this material can be useful in many food-related industrial aspects where prevention of oxidative damage or free radical formation is involved. Therefore, food quality, shelf-life extension, and intelligent packaging could be improved or maintained by developing these characteristics [2]. In general, the scale-up production of valuable bioactive products from agro-industrial wastes is generally troublesome. The main reason is the large gap between laboratory and large-scale production, and the development of laboratory standard operating procedures for kilogram-scale production is challenging nowadays. Also, the synthesis of derivatives requires chemically pure bioactive compounds. Thus, the elaboration of simple, rapid, cost-saving, and eco-friendly extraction, purification, and analytical procedures for routine preparation or semi-industrial production of high-purity natural bioactive compounds including quercetin can be considered a relevant way of solving the problem. Extraction is one of the essential stages in investigating desired bioactive compounds, recycling agro-industrial wastes and their application in the nutraceutical and pharmaceutical industries. One of the pioneering conventional methods for extraction is dependent on the polarity of the target compound and solvent systems. Solvents such as a mixture of water and ethanol, water, ethanol, and methanol have been most commonly utilized to obtain extractable compounds from onion peels. Recently, to reduce the consumption of solvents, make the process eco-friendly, and at the same time enhance the efficiency of extraction, the use of different methods such as microwave-assisted extraction (MAE), ultrasound-assisted extraction (UAE), pulsed electric field (PEF) extraction, enzyme-assisted extraction (EAE), supercritical fluid extraction (SFE), subcritical water extraction (SWE), and pressurized liquid extraction (PLE) has been encouraged. With respect to extractions of bioactive compounds from onion peel or waste, various methods, such as conventional extraction and nonconventional extraction methods, such as SWE, UAE, and MAE, have been reported by several authors. The usage of conventional solvents for extraction leads to adverse effects on the environment, considering their volatility, nondegradable nature, and toxicity. These methods are time- and/or solvent-consuming, are not selective, and are targeted at total phenols rather than quercetin. However, these green processes reduce the harmful impacts on the environment by minimizing or eliminating the use of solvents and improving extraction efficiency [14-23]. The aim of the present work was to develop various alternative methods for obtaining high-purity quercetin from onion peel that would be easy to perform, fast, effective, selective, reproducible, time- and energy-saving, eco-friendly, and cheap without the use of toxic solvents. The concept of the present work included the development of a method that would not only to allow us to obtain the target quercetin in laboratory conditions but also that would be capable of and appropriate to be transferred to an industrial scale, and contribute to the application of onion peel as an agro-industrial waste. The various protocols capable of efficiently reprocessing onion peel, combined with the purification dry column vacuum chromatography (DCVC) and analytical spectrophotometric procedures for obtaining quercetin in the form of the extracted dried product, are proposed in the present paper by the authors. The different extraction techniques, solvents, sequence of stages, temperature range, and extraction time were investigated during the experiment. A certain part of the work is devoted to an extensive discussion of the economic feasibility of each developed procedure, evaluated according to various parameters. As a result, there was determined various methods intended for use, met our goals and the predetermined target profile.
Materials and Methods Materials The ethyl acetate, n-hexane, acetone, ethanol, methanol, petroleum ether, white spirit, sodium sulfate, Merck silica Gel 60 – 0.015-0.040 mm - №1.15111.1000 Celite® 545 particle size 0.02-0.1 mm were purchased from Merk. The certified analytical reference standard of quercetin dihydrate (89.1% calculated on anhydrous quercetin) was purchased from United States Pharmacopoeia (USP). Instrumentation The Milli Q Adventage A10 purification system (Millipore, France), dual-frequency ultrasonic bath DW-5200DTS (China), Vortex-Genie™ 2 Mixer (USA), Magnetic Stirrer IKA C-MAG HS 10 (China), pH-meter Hanna Instruments HI 2211 (USA), analytical balance ALX-210 (USA), Biobase small capacity rotary evaporator (China), GFL water bath (Germany), Hermle Z200A centrifuge (Germany), laboratory mill SM-450C were used for sample preparation. The spectrophotometric analysis was performed using UV-Vis spectrophotometry UV-1900i Shimadzu (Japan). All the measuring equipment was appropriately calibrated. Methods The ultrasound-assisted and boiling with heater extraction techniques were used to extract the target compound from onion peel. 20 g of the powdered dried sample of onion peel was transferred to a 250 mL round-bottomed (conic) flask. Various volumes of the following extraction solvents – ethyl acetate, acetone, ethanol and purified water were used. Experiments were carried out by ultrasonication at 40 kHz and boiling with a heater at different times and various temperatures. The obtained organic extracts during the extraction process were dried through sodium sulfate. After sufficient evaporation of the solvent, 3 g of celite was added to the wet sample. In order to evaporate the solvent during the extraction process, a rotary evaporator at 30-50°C under reduced pressure was used to give a dried homogeneous mixture. The obtained dried solid sample was used for the purification stage. The purification of the obtained dried extract was performed using the DCVC with ethyl acetate/hexane, ethyl acetate/petroleum ether and ethyl acetate/white spirit as an eluent solvent, which was composed of a cylindrical sintered glass funnel (height – 10 cm, diameter – 4 cm), a separating funnel, and a glass joint connecting these two with a sidearm to apply a vacuum and aspirator pump. Merck Silica Gel 60 – 0.015-0.040 mm – Merck № 1.15111.1000 was used as an adsorbent (height – 7 cm). The reference standard of quercetin dihydrate was dissolved in methanol and diluted with the same diluent to obtain a standard solution at 0.004 mg/mL concentration. In order to prepare the test solution, the dried extracted product was dissolved and diluted in the same manner as the standard solution to obtain the same concentration (100 %). The obtained solution was filtered through a 0.45 µm through 0.45 µm polyvinylidene difluoride (PVDF) microporous membrane filter. The quantification was performed using the external standard method. Methanol was used as a blank solution. The absorbances of the standard and the test solutions were measured at a wavelength of 375 nm. The UV-Vis spectra of solutions were scanned in the wavelength range of 190–600 nm. Calculations The concentration of quercetin – CS, mg/mL in the test solution was calculated by the following equation (1): where, AbsSt – the absorbance of the standard solution; AbsS – the absorbance of the standard solution; WSt – the weight of the standard of quercetin dihydrate reference standard, mg; D – the dilution factor of the standard; P – the purity of the standard of quercetin dihydrate calculated on anhydrous basis, %. The percentage content of quercetin (purity) – X, % in the extracted product was calculated in equation 2: where, WS – the weight of the extracted product sample, mg taken for analysis; VS – the dilution of the extracted product sample, mL. The percentage content of quercetin – x, %, in the onion peel (the percentage yield of the extraction procedure) was calculated in equation 3:where, m – the weight of the dried sample of onion peel taken for the extraction, mg; W – the weight of the extracted product, mg; where, M – the weight of the dried sample of onion peel taken for the extraction, g. The percentage recovery - R, % was calculated by the following equation (5):where, Wd – the determined amount (added and indigenous) of quercetin, mg in the dried sample of onion peel added analytical standard; Wa – the amount of standard of quercetin, mg to the dried sample of onion peel, mg. The similarity factor – Sf, % for two standard solutions was calculated by the following equation (6):where, AbsSt1 – the absorbance obtained with the standard solution I; WSt1 – the weight of the standard for the standard solution II, mg; AbsSt2 – the absorbance obtained with the standard solution I; WSt2 – the weight of the standard for the standard solution II, mg.
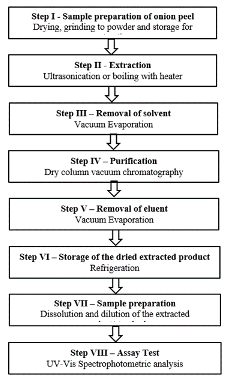
Results and Discussion Extraction procedures were developed by taking into account the following factors: the selectivity and yield of the extraction procedure, and the purity of the extracted product. To enhance the efficiency of the extraction process and increase the recovery of target compound for extracting compound from onion peel, various critical parameters – ultrasonic power, extraction time, solvent volume, solvent nature, temperature, and the amount of onion peel – were investigated and selected. The effect of key parameters for quercetin UAE extraction was evaluated by varying the temperature (25°C, 50°C, 60°C), the extraction time (30-60 minutes) and the extraction solvent (ethyl acetate, acetone, ethanol and water). The extraction temperature of 50°C was determined to be the optimal operating temperature for volatile organic solvents, 60°C for purified water. An increase in the temperature slightly increases the yield of the target product but drastically decreases selectivity; lowering the temperature decreases the yield of extraction. The optimal extraction time for UAE was 1 hour. The selectivity decreased significantly if the extract was stored after the extraction, even for 30 minutes. The priority of solvents toward selectivity was arranged as follows: ethyl acetate>acetone>water>ethanol. The solvent priority in the evaporation stage from a technological point of view was ethyl acetate>acetone>ethanol. Eleven extraction methods with their purification procedures were developed and established in the form of eleven standard protocols described below. These methods can be represented in the general scheme given in Figure 1. For purification, the DCVC method was used, which was a fast and efficient alternative technique.
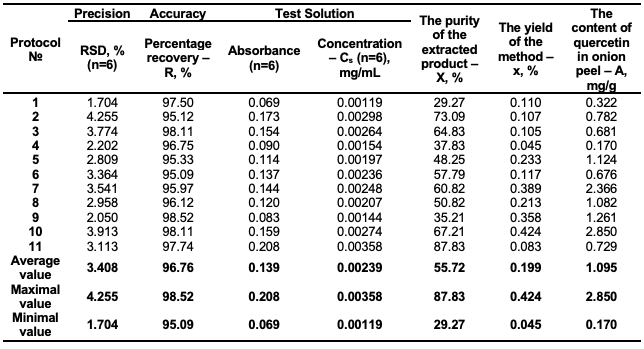
The elution gradient was initially set to be 100% hexane, petroleum ether, white spirit and the percentage of ethyl acetate was gradually increased with 5% from 0 to 100%; The volume of each eluent portion was 20 mL; initially, the obtained fractions (1-20 fractions) were collected in 25 mL test tubes. The UV-Vis spectrophotometer was then used to detect the presence of quercetin in each fraction. Fractions containing the same compounds were then combined, and the solvent evaporated to dryness on a rotary evaporator. The eluent priority in the DCVC purification stage was ethyl acetate/white spirit>ethyl acetate/petroleum ether/hexane>ethyl acetate/hexane [19,24]. Protocol 1: The extraction procedure: 20 g of dried onion peels were ground, weighed and placed in a conic flask; 200 mL of ethyl acetate was added and ultrasonicated for 30 minutes at 25-30°C. The extract was stored for half hour, filtered on filter paper; 3 g of celite was added to the filtrate and solvent was evaporated on a rotary evaporator. The obtained powder was stored in a refrigerator for further purification. The DCVC purification: eluent system – ethyl acetate/hexane with gradient 5%. Protocol 2: The extraction procedure: 20 g of dried onion peels were ground, weighed and placed in a conic flask; 200 mL of ethyl acetate was added and ultrasonicated for 1 hour at 25-30°C. The extract was stored for 2 hours, filtered on filter paper; 3 g of celite was added to the filtrate and solvent was evaporated on a rotary evaporator. The obtained powder was stored in a refrigerator for further purification. The DCVC purification: eluent system – ethyl acetate/hexane with gradient 5%. Protocol 3:The extraction procedure: 20 g of dried onion peels were ground, weighed and placed in a conic flask; 200 mL of acetone was added and stored for 24 hours at 25-30°C. The extract was filtered on filter paper; 3 g of celite was added to the filtrate and solvent was evaporated on a rotary evaporator. The obtained powder was stored in a refrigerator for further purification. The DCVC purification: eluent system – ethyl acetate/hexane with gradient 5%. Protocol 4:The extraction procedure: 20 g of dried onion peels were ground, weighed and placed in a conic flask; 200 mL of ethanol was added and ultrasonicated for 1 hour at 25-30°C. The extract was stored for 2 hours and filtered on filter paper; 3 g of celite was added to the filtrate and solvent was evaporated on a rotary evaporator. The obtained powder was stored in a refrigerator for further purification. The DCVC purification: eluent system – ethyl acetate/hexane with gradient 5%. Protocol 5:The extraction procedure: 20 g of dried onion peels were ground, weighed and placed in a conic flask; 400 mL of purified water was added and ultrasonicated for 1 hour at 60°C. The extract was stored for 30 minutes. Filtered and extracted with ethyl acetate (50×3mL). Ethyl acetate phase was dried for 1 hour and 3 g of celite was added. Solvent was evaporated on a rotary evaporator, and the obtained powder was stored in a refrigerator. This procedure had been repeated on the same sample of onion peel. The obtained powdered samples were combined for further purification. The DCVC purification: eluent system – ethyl acetate/petroleum ether with gradient 5%. Protocol 6:The extraction procedure: 20 g of dried onion peels were ground, weighed and placed in a conic flask; 200 mL of ethyl acetate was added and ultrasonicated for 1 hour at 25-30°C. The extract was filtered on filter paper; filtrate was evaporated, and the powder stored in a refrigerator. 200 mL of ethyl acetate was added to the same sample of onion peel and ultrasonicated for 1 hour at 50°C. The extract was stored 2 hours filtered on filter paper; filtrate was evaporated. The powdered samples were combined, 100 mL of purified water was added, and the mixture was ultrasonicated for 1 hour at 25-30°C, filtered, and the filtrate was extracted with ethyl acetate. Solid phase was dissolved in ethyl acetate. Ethyl acetate phases were combined, 3 g of celite was added, solvent was evaporated on a rotary evaporator, and crude quercetin was stored in a refrigerator for further purification. The DCVC purification: eluent system – ethyl acetate/hexane with gradient 5%. Protocol 7:The extraction procedure: 20 g of dried onion peels were ground, weighed and placed in a conic flask; 800 mL of purified water was added and heated 1 hour at 90-100°C. The extract was stored for 30 minutes, then filtered and again stored for 72 hours. The formed precipitate was filtered on filter paper and filtrate was extracted with ethyl acetate (50 mL×3). Ethyl acetate phase was dried on Na2SO4 for 1 hour and 3 g of celite was added. Solvent was evaporated on a rotary evaporator, and the obtained powder was stored in a refrigerator for further purification. Precipitate was immersed in a conic flask, 150 mL of ethyl acetate was added and ultrasonicated for 30 minutes at 25-30°C, the solution was stored for 30 minutes, and filtered on filter paper. 3 g of celite was added, solvent was evaporated on a rotary evaporator, and the powder was stored in a refrigerator for further purification. The DCVC purification: eluent system ethyl acetate/petroleum ether with gradient 5%. Protocol 8:The extraction procedure: 20 g of dried onion peels were ground, weighed and placed in a conic flask; 400 mL of purified water was added and ultrasonicated for 1 hour at 60°C. The extract was stored for 30 minutes, then filtered and extracted with ethyl acetate (100 mL×3). Ethyl acetate phase was dried for 1 hour and 3 g of celite was added. Solvent was evaporated on a rotary evaporator, and the obtained powder was stored in a refrigerator for further purification. The DCVC purification: eluent system – ethyl acetate/white spirit with gradient 5%. Protocol 9:The extraction procedure: 20 g of dried onion peels were ground, weighed and placed in a conic flask; 400 mL of purified water was added and ultrasonicated for 1 hour at 60°C. The extract was stored for 30 minutes, then filtered and extracted with ethyl acetate (50 mL×3). Ethyl acetate phase was dried for 1 hour and 3 g of celite was added. Solvent was evaporated on a rotary evaporator, and the obtained powder was stored in a refrigerator. The DCVC purification: eluent system – ethyl acetate/hexane with gradient 5% for fraction 1-10 and gradient 2.5% for fraction 11-17. Protocol 10:The extraction procedure: 20 g of dried onion peels were ground, weighed and placed in a conic flask; 400 mL of purified water was added and boiled for 30 minutes. The extract was stored for 30 minutes and filtered on filter paper. The filtrate was extracted with ethyl acetate (50 mL×3). Ethyl acetate phase was dried on Na2SO4 for 1 hour and 3 g of celite was added. Solvent was evaporated on a rotary evaporator, and the obtained powder was stored in a refrigerator for further purification. The DCVC purification: eluent system – ethyl acetate/hexane with gradient 5% for fraction 1-10 and gradient 2.5% for fraction 11-17. Protocol 11:The extraction procedure: 20 g of dried onion peels were ground, weighed and placed in a conic flask; 200 mL of ethyl acetate was added and ultrasonicated for 1 hour at 25-30°C. The extract was filtered on filter paper; 3 g of celite was added to the filtrate and solvent was evaporated on a rotary evaporator, and the obtained powder was stored in a refrigerator for further purification. The DCVC purification: eluent system – ethyl acetate/hexane with gradient 5% for fraction 1-10 and gradient 2.5% for fraction 11-17. Six individual extractions were performed according to each protocol. Each method with analytical procedure was verified with respect to the following performance parameters: specificity, precision and accuracy according to the appropriate guideline and the methodologies reported by the authors [25-28]. All the extracted dried products containing quercetin, obtained with the developed methods, were analyzed using UV-Vis spectrophotometric procedure and measured absorbances (at 375 nm) of standard and tests solutions. Accordingly, the concentrations of quercetin, expressed in mg/mL in test solutions – Cs, the percentage content of quercetin (purity) – X, % in the extracted product, the yield of the extraction procedure – x, % and the content of quercetin in onion peel, mg per 1 g of the waste material – A were evaluated by calculating equations 1-4, respectively. The results are given in Table 1.
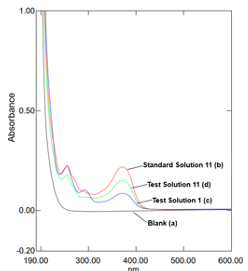
The specificity test of the analytical procedure was checked by scanning the background control - blank (methanol), standard and test solutions in the wavelength range of 190–600 nm. According to the obtained UV-Vis spectra, there was no interference, and the blank solution had no absorbance at 375 nm. There was a very strong spectral similarity between standard and test solutions.
Figure 2 depicts the overlapping UV-Vis spectra obtained with the blank, standard solution and test solutions scanned in the wavelength range of 190-600 nm. Hence, this analytical spectrophotometric procedure could specifically measure the absorbances of the analytes. The precision of each method was estimated by preparing the two standard solutions and six test solutions according to each protocol. This parameter was checked by calculating the RSD of six determined concentrations (mg/mL) of quercetin (Cs) in the test solutions (acceptance criteria: ≤5.0%). The similarity factor – Sf, % between the absorbances obtained with two standard solutions was calculated by the equation 5 and should be within 98.0 %-102.0% (acceptable criteria). The calculated value of the Sf was 100.11%. The results of the precision test are given in Table 1 which show that all the developed procedures have a good precision. The accuracy test was assessed by performing recovery studies using the standard addition method by spiking known amount of analytical standard in the test solution at 150% of quercetin. The spiked test solution was prepared according to the following procedure: the analytical standard of quercetin was mixed with the dried and powdered sample of onion peel to obtain 150% of quercetin in the spiked test solution before starting the extraction procedure. The accuracy was expressed as a percentage recovery which was the percentage of standard compound recovered from the spiked test solution (sample + standard) and calculated using equation 6. The recovery – R, % should be within 95.0-105.0% (acceptance criteria). The results given in Table 1 show that all eleven procedures have a good recovery rate. Based on the analytical results, all eleven developed methods described in the above-mentioned protocols are specific, characterized by good precision and accuracy and respectively considered to be verified procedures. As the results show, the determined percentage contents of quercetin in the extracted products are different. Protocol 11 is characterized with the highest purity (87.83%) of the extracted product, and the lowest purity (29.27%) is observed in case of protocol 1. The UV-Vis spectra (Figure 3) confirms that the highest purity of the extracted product was obtained with protocol 11, and its spectral similarity to the standard is equal to 0.999.
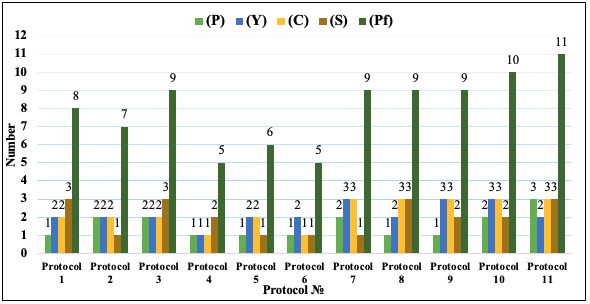
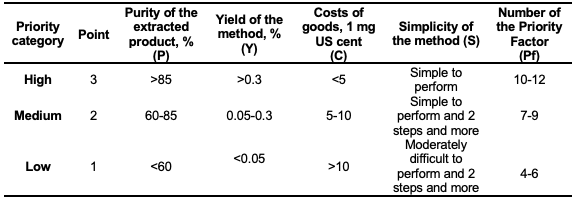
A different picture is observed if we compare the developed procedures in terms of the extraction yield. The highest yield (0.424%) was obtained with protocol 10, although the purity of the product is high and equal to 67.21% but relatively lower than the result obtained with protocol 11. Despite the acceptable yields of the extraction observed, the methods described in protocols 1, 4, 5, 6, 8, and 9 do not provide the possibility of obtaining high-purity quercetin; therefore, it is necessary to use additional purification procedures of the obtained product. The extracted product with the highest purity of quercetin can be obtained with protocols 2 and 11, and the highest extraction yield of quercetin can be obtained with protocols 7, 9, and 10. The extraction procedures described in protocols 2, 10, and 11 are characterized by an acceptable yield, and the purity of the extracted products obtained using them is relatively higher. Therefore, these three proposed procedures are eco-friendly, effective, and selective extraction methods based on ultrasonication that can be used successfully in laboratory conditions. To evaluate feasibility and select an optimal method for preparative purposes in industrial scale, the following four parameters were used: the purity of the extracted product (P), yield of the method (Y), costs of goods (C), and simplicity of the method (S). The evaluation was carried out using a quantitative method using the mentioned parameters. Each parameter had a 3-point scale (Table 2). Finally, the number of the priority factor (Pf) was calculated by the simple equation [Pf = (P)+(Y)+(C)+(S)]. The higher the calculated number of the priority factor, the higher the priority and feasibility of the developed method. The results of the feasibility evaluation of the methods are given in Figure 3. The highest number of the priority factor was observed in case of protocol 11; additionally, the purity of the extracted product was higher (87.83 %). Therefore, the method is appropriate and suitable to be used for preparative purposes and to be transferred from laboratory conditions to industrial scale.
Based on the analytical data, the percentage contents of quercetin (the purity of the extracted product) – A, expressed in mg per 1 g of the dried and powdered samples of onion peel, vary from 0.170 mg/g to 2.850 mg/g, calculated on the anhydrous basis. The purity is equal to the purity of the standard, which was also confirmed by the presented UV spectrum (Figure 2).
Conclusion It has been shown that onion peel is a rich source of quercetin through the application of advanced eco-friendly and low-cost extraction techniques with the use of non-toxic solvents. The proposed three different, effective, eco-friendly, reproducible, and selective ultrasound-assisted extraction methods, combined with suitable and verified analytical spectrophotometric procedures for obtaining quercetin from onion peel, are alternative laboratory methodologies that provide a high-quality target quercetin in the form of the dried powdered extracted product. In addition, these proposed methods can be successfully used by scientific and quality control laboratories to quantitatively determine quercetin in onion peel and other phytoextracts. Also, there is proposed a fast, effective, selective, reproducible, time- and energy-saving, eco-friendly, and cheap extraction method based on the feasibility evaluation that should be suitable for scale-up and can be transferred to the industry in order to obtain quercetin in kilogram-scale production from onion peel as an agro-industrial waste material. Further studies should be aimed at standardizing and validating the proposed method in order to obtain a pilot product as an ingredient for later development of a commercial production line of food or nutraceutical formulations.
Conflicts of Interest The author(s) declare(s) that there is no conflict of interest regarding the publication of this paper. References
|
||||||||||||||||||||||||||||||||||