|
Introduction Plants are one of the most important sources of medicines. The medicinal plant is very old. The writings indicate the therapeutic use of plants as far back as 4000-5000 B. C. The Chinese were the first to use the natural herbal. In India, however, the earliest references of the usage of plants as medicine appear in Rigveda, which is supposed to have been penned between 3500-1600 B. C. Later, the properties and healing uses of medicinal plants were analyzed in detail and recorded empirically by the ancient physicians in Ayurveda (an indigenous system of medicine), which is a basic institution of ancient medical science India [1]. O. sanctum, “the holy place”, has been experienced from as early as the V edic period, usually known as “Tulsi”, belonging to the family of Lamiaceae. The work is kept sacred by Indians all over the globe as it is an herbaceous plant that is used for religious purposes in accession to its great medicinal values. The plant is an aromatic bushy shrub. It springs up all over India, growing up to 2000 meters in the mounds. It grows in houses, temples, and gardens. Leaves have a sweet, clove-like scent. There are many varieties of this species. It presents in a range of foliage colours (royal and green), leaf shapes and grains, bloom colors, heights and fragrances. The blooms are tiny, purple or white and inflorescence is a long spike. Of all the Ocimum species, O. sanctum is most widely studied for its pharmacological properties. Traditionally, the leaves are used to treat bronchitis, and gastric, skin and hepatic disorders. Diverse pharmacological properties of O. sanctum (for example antidiabetic, immunostimulant, antioxidant, cardioprotection, antifungal) have attracted entrepreneurs to set eyes on this plant. Leaf extract of O. sanctum showed significant ability to scavenge free radicals. The industry sector perceives O. sanctum not just as a spice used in household kitchens. They, too, view it as a potential lead in the production of drugs that are important to satisfy the insatiable demands of the population. These drugs would be cost effective and quite suitable to millions of our masses, as this herb and its remedies have been in use from ancient times [2]. The leaves contain an essential oil which is composed of eugenol, carvacrol, methylchavicol, limatrol, and caryophylline. The seeds contain oil composed of fatty acids and sitosterol. The roots contain ursolic acid and n-triacontanol. Eugenol – its methyl ether, nerol, caryophyllene, terpinen4-decyladehyde, selinene, pinenes, camphene and α-pinene have been identified in essential oil. To boot, it also contains rosmarinic acid, thymol, linalool, and methyl chavicol and citral [3]. Because of remarkable biological activities of this plant, our aim is to predict the molecular properties and to evaluate the bioactivity scores using M olinspiration [4-6] Materials and Methods
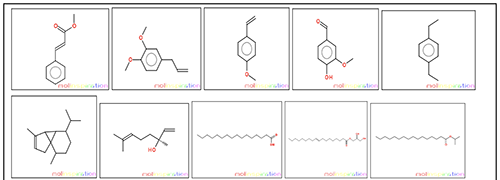
Structures of all the ten compounds reported from O. sanctum were taken from the literature and their structures were drawn using online M olinspiration software (www.molinspiration.com) (Figure 1) for calculation of molecular properties (Log P, t otal polar surface area, number of hydrogen bond donors and acceptors, molecular weight, number of atoms, number of rotatable bonds, etc.) and prediction of bioactivity score for drug targets (GPCR ligands, kinase inhibitors, ion channel modulators, enzymes and nuclear receptors) [4]. The bioactivity score and drug- likeness properties of the all the ten compounds were compared. Prediction of bio-activity
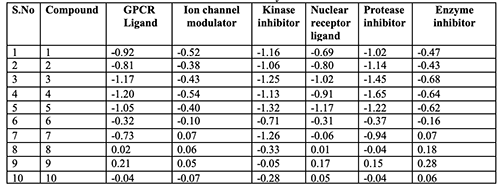
2. Bio-activity scores of the nine alkoxy towards GPCR ligand, ion channel modulator, nuclear receptor ligand, kinase inhibitor, protease inhibitor and enzyme inhibitors are given in Table 2.
Lipinski’s Rule: Lipinski’s rule of five, commonly known as the Pfizer’s rule of five or simply the Rule of five, is a regulation of thumb to estimate drug- likeness or to identify a chemical compound with a certain pharmacological or biological activity that has properties that would make it a likely orally active drug in humans. The principle was designed by Christopher A. Lipinski in 1997. The rule expresses molecular properties vital for a drug’s pharmacokinetics in the human body, including their absorption, distribution, metabolism and elimination (ADME) c omponents of the Lipinski’s rule [5,6]. Lipinski’s rule states:
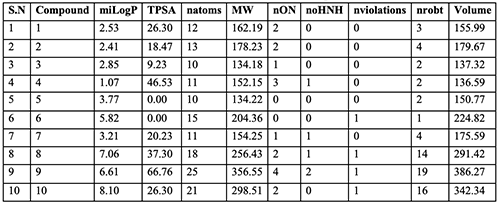
Molinspiration Molinspiration, a web-based software, was used to obtain parameters such as MiLogP, TPSA, and drug- likeness. MiLogP is estimated by the methodology developed by Molinspiration as a sum of fragment-based contributions and correction factors. The MiLog P parameter is applied to check good permeability across the cell membrane. TPSA is related to the hydrogen bonding potential of the compound. Computation of volume developed at Molinspiration is based on group contributors. Number of rotatable bonds measures molecular flexibility. It is a very good descriptor of absorption and bioavailability of drugs. Through the drug- likeness data of a particle the molecular properties and structure features can be checked with regard to known drugs. Bioactivity score Bioactivity of the drug can be found out by estimating the activity score of GPCR ligand, ion channel modulator, nuclear receptor ligand, kinase inhibitor, protease inhibitor, and enzyme inhibitor. All the parameters were determined with the aid of Molinspiration drug-likeness score online (www.molinspiration.com). The calculated drug- likeness score of each compound was compared with the specific bodily process of each compound, and the results were compared with Naringenin. For organic molecules the probability is if the bioactivity score is (> 0), then it is active; if (-5.0-0.0), then moderately active; if (< -5.0), then inactive. Results and Discussion
The ten oil constituents obeyed the Lipinski’s rule of five and showed good drug- likeness scores. MiLog P values of these oily compounds were found to be < 5 (1.07-5.82 for compounds 1 to 7), indicating their good permeability across the cell membrane. All the derivatives were found to have TPSA and will be below 160Å2 (100.13), molecular weight < 500, n o. of hydrogen bond donors ≤ 5, n o. of hydrogen acceptor ≤ 10, n-violations 0, number of rotatable flexible bonds >5.
The bioactivity scores of the ten compounds have shown the following observations:
Conclusion In conclusion, ten compounds show highly active to moderate bioactivity score. All compounds obey Lipinski’s rule for drug- l ikeness activity of the molecules. References
|
||||||||||||||||||||||||