|
Introduction In 1791, Luigi Galvani discovered electrical activity in the nerves of the frogs that he was dissecting. He thought that electricity was of animal origin and could be found only in living tissues [1]. A few years later, in 1800, Alessandro Volta discovered that electricity could be produced through inorganic means. In fact, by using small sheets of copper and zinc and cloth spacers soaked in an acid solution, he built a battery – the first apparatus capable of producing electricity [1]. Electricity has a central role in our lives and to this day electrochemistry is a standard course of study [1]. Today batteries provide the power for an amazing variety of devices, everything from flashlights to robots, computers, satellites and cars. Inventors and researchers continue to improve the battery, designing batteries that last longer and that are friendlier to our environment [2]. Energy consumption throughout the world, but particularly in industrialized societies, has been steadily increasing. Much of the energy consumed, 97% in the case of the UK, comes from non-renewable sources [3]. The present use of carbon-based non-renewable energy is unsustainable because of the effect of the resultant carbon dioxide (CO2) emissions on the global climate [3]. Reduction in demand must be part of the solution but alternative energy sources must also be developed. All energy sources come with environmental penalties, whether from the construction of dams and barriers or from the impact of renewable sources such as wind on rural landscapes, but these impacts must be balanced against the necessity of developing low-carbon sources that are both economically viable and, also, secure [3]. Most fruits can conduct electricity because they contain water with various substances dissolved in it. How much electricity they conduct depends on how much water there is and what is dissolved in it. Most fruits have some acid which results in many free ions that allow the flow of electricity, so acidic fruits are quite good conductors of electricity (that is, with low resistance). Lemons and limes are acidic fruits. They contain citric acid which makes them good conductors [4,5]. Organic acids are of great significance in plants. As intermediates in the metabolic processes of the fruits, these acids are directly involved in growth, maturation, and senescence. Fruit juices have a low pH, because they contain high levels of organic acids. The total acid content varies widely, from approximately 0.2% in pear juice to 0.8% in lime. Some of the major acids in fruits include citric, malic, and tartaric acids [6]. Many experiments were carried out to generate electricity from vegetables and fruits. Erika Lindstorm did her experiment which was aimed at finding out which fruit would generate enough electricity to light a light bulb and to discover which fruit would light the bulb the longest. Copper and zinc electrodes were used with a multimeter; the fruit samples were Sunkist lemons, Texas Rio Star grapefruit, hot house tomatoes, Kiwis, and California Navel oranges. After several tests she discovered that the light bulb would not light up with any of the fruits using any of the variables due to the high resistance of the light bulb and because the fruit did not produce enough current [7]. Creating a battery from a lemon is a common project in many science text books. Batteries consist of two different metals suspended in an acidic solution. Zinc and copper work well as the metals and the citric acid content of a lemon will provide the acidic solution. Batteries like this will not be able to run a motor or energize most light bulbs. It is possible to produce a dim glow from an LED [4]. Potato was used with zinc and copper electrodes as a battery [8]. In another advanced research project, Professor Chungpin Hovering Liao of National Formosa University in Taiwan has created the world’s first chlorophyll organic battery (http://www.ecofriend.org/entry/eco-tech-any-liquid-is-good-for-the-world-s-first-chlorophyll-battery/; http://www.goodcleantech.com/2008/10/chlorophyll_organic_battery_ru.php). The battery can use any liquid, even urine, to power up. It doesn’t take much time to start juicing the battery, either. Within 10 seconds of being doused with liquid, the battery starts providing power [9]. Christopher J. Love and other workers studied the source of sustained voltage difference between the xylem of a potted Ficus benjamina tree and its soil. They have measured sustained differences of 50–200 mV between the xylem region of a Faraday-caged, intact, potted Ficus benjamina tree and its soil, as well as between its cut branches and soils and ionic solutions standardized to various pH values. Using identical platinum electrodes, no correlation between voltage and time of day, illumination, sap flow, electrode elevation, or ionic composition of soil was found, suggesting no direct connection to simple dissimilar-metal redox reactions or transpirational activity. Instead, a clear relationship between the voltage polarity and magnitude and the pH difference between xylem and soil was observed. They attributed these sustained voltages to a biological concentration cell likely set up by the homeostatic mechanisms of the tree. The proposed practical applications of these findings, beyond monitoring pH changes, include a wide variety of trickle chargers for niche, low-power, pulsed, off-grid distributed systems – such as forest fire detectors; environmental sensors; and ‘‘smart dust’’ or mesh networked devices – which drastically decrease the need for in-the-field battery changes. Interestingly, ionic flows through micro-fluidic circuits have already been investigated as viable sources of microwatt level electrical power [10].
Aims of the Study There is currently an upsurge of interest in finding renewable energy resources due to the international rise in the price of oil, which has negative effects on the economies of industrial as well as developing countries. Moreover, the environmental pollution due to the combustion of fuel is one of the most serious issues worldwide. All countries tend to solve the oil bill problem and to reduce environmental pollution to control global warming by searching for alternative energy resources like wind power, water power, nuclear power, etc. Jordan is suffering from inconveniently high oil prices: that was one of the most important motives to search for new renewable energy resources. As a preliminary study that precedes the current project, it was noticed that the cactus stems generate potential difference close to 1 volt. This exciting result encouraged me to carry out further experiments to test the ability to generate electricity from cactus due to the following reasons: (i) the ability to grow it in any location in Jordan and worldwide, even in arid lands and deserts; (ii) its high rate of growth; (iii) the photosynthesis occurs in all its parts which makes it easy to connect the electrodes to the low parts of the cactus stem; and (iv) the dependence on citric acid production along with other acids during photosynthesis which makes it different from the lemon and potato battery or any other kind of citrus batteries. The current research project was aimed to achieve the following objectives:
Experimental Sample collection Cactus plates (Opuntia) were collected from Kufr’ Awan, Irbid district, in the Northern part of Jordan during January-March 2019. All the samples were collected from a house garden of red soil type. Materials and Methods Copper electrodes (stripe), magnesium electrodes (stripe), galvanized nails, cactus (Opuntia) plates (Figure 1), and a multimeter, 3- & 5-mm diameter LEDs were used for the experiments.
To compare the generated potential difference of cactus (Opuntia) pieces using different electrodes of copper, magnesium, and zinc. A cactus (Opuntia) plate was cut into pieces of ~ 4 × 4 cm, then a copper stripe and zinc were inserted into the cactus pieces to a distance of 2 cm; after that, the electrodes were connected to a multimeter using wires. The readings were then recorded every 5 min for 15 min. The experiment was carried out in triplicate. The above described experiment was then repeated by replacing the galvanized nail electrode with magnesium electrode. To determine the suitable number of cactus pieces that are enough to light 3 & 5 mm LED lamps, a cactus (Opuntia) plate was cut into pieces of ~ 4 × 4 cm, then a copper stripe and a magnesium stripe were inserted into one cactus piece to a distance of 2 cm; after that, the electrodes were connected to light 3 & 5 mm LED lamps using wires. The same steps were repeated by changing the connected number of cactus pieces in series from 1 to 6. To measure the potential difference for 4 pieces of cactus connected in series, a cactus plate was cut into 4 equal pieces (~ 4 × 4 cm), then copper and magnesium stripes were inserted into the cactus pieces to a distance of 2 cm (the area of the electrode is irrelevant to the generated potential difference). The four pieces of cactus were then joined together so that the copper of the first piece was connected to the magnesium stripe of the second piece. The terminal electrodes of copper and magnesium were then connected to a multimeter as in Figure 2.
To measure the potential difference for 8 pieces of cactus connected in series, a cactus plate was cut into 8 pieces of equal area (~ 4 × 4 cm), then copper strips and galvanized nails were inserted into the cactus pieces to a distance of 2 cm (the area of the electrode is irrelevant to generated potential difference). The 8 pieces of cactus were then joined together so that the copper of the first piece was connected to the magnesium stripe of the second piece. The terminal electrodes of copper and galvanized nail were then connected to a multimeter. To determine the potential difference variations with time, a cactus plate was cut into 13 pieces of equal size (~ 4 × 4 cm), then copper and magnesium stripes were inserted into the cactus pieces to a distance of 2 cm (the area of the electrode was irrelevant to the generated potential difference). Using wires, the 13 pieces were then joined in a series so that the copper of the first piece was connected to the magnesium stripe of the second piece, then the terminal electrodes of copper and magnesium were connected to a multimeter. The potential difference variations over 40 hours were then recorded. To determine the current variations with time, a plate of cactus was cut into 13 pieces of equal size (~ 4 × 4 cm), then copper and magnesium stripes were inserted into the cactus pieces to a distance of 2 cm (the area of the electrode was irrelevant to the generated potential difference). Using wires, the 13 pieces were then joined in a series so that the copper of the first piece was connected to the magnesium stripe of the second piece, then the terminal electrodes of copper and magnesium were connected to a multimeter and LED lamp in series. The current variations over 40 hours were then recorded. To determine how long the LED lamp can continue to light up after 40 hours of continuous lighting, as well as determine the potential difference and current variations with time after 40 hours, a plate of cactus was cut into 6 pieces of equal size (~ 4 × 4 cm), then copper and magnesium stripes were inserted into the cactus pieces to a distance of 2 cm. Using wires, the 6 pieces were then joined in a series, then the terminal electrodes of copper and magnesium were connected to a multimeter and LED lamp in series to record the current values. When the LED lamp was replaced with a multimeter to record the potential difference values, the LED lamp lit up for over 4 days (100 hours). To compare the generated potential difference of cactus (Opuntia) plates directly from the tree, different electrodes of copper, magnesium, and galvanized nails were used. Cactus (Opuntia) plates were selected to be in the middle of the branches (not new plate), then a copper stripe and a galvanized nail were inserted into the cactus pieces to a distance of 2 cm; after that, the electrodes were connected to a multimeter using wires. The readings were then recorded every 5 min for 15 min. The experiment was carried out in triplicate. The above described experiment was then repeated by replacing the galvanized nail electrode with a magnesium electrode. To determine the ability of using cactus (Opuntia) extract to generate electricity, pieces of cactus plate were squeezed to collect their extract in a dish. Then the prepared electrochemical cell, which was built by enveloping a Mg electrode with filter paper and Cu wire, was soaked in cactus extract for about two minutes to become saturated. The same procedure was carried out for another 5 cells. After that, all 6 cells were connected in series to measure the generated potential difference and current variations for a period of 6 hours.Results and Discussion The data collected from the cactus experiments are as the following: Table 1 shows the effect of using different electrodes on the generated voltage values.
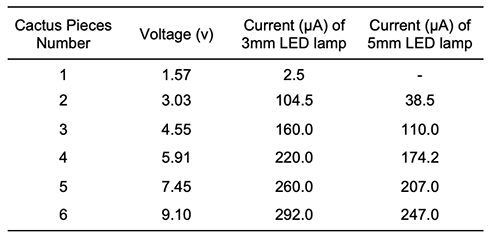
By calculating the voltage average of the (Zn/Cu) cactus cell which was 0.995 V and the voltage average of the (Mg/Cu) cactus cell which was 1.64 V. It was clear that using the Zn/Cu electrode enhanced the potential difference for one cactus electrochemical cell by 39%. From the experiments which were carried out to determine how many pieces of cactus were needed to light 3 & 5 mm LED lamps, the results presented in Table 2 show that one piece was enough to light up the 3 mm LED lamp, whereas a minimum of two pieces of cactus were required to light up the 5 mm LED lamp. This difference refers to the LED lamp resistance which is increased with the increment of the lamp size. Beside that, the data showed that as the number of the cactus pieces increased, the voltage, the current and the LED lamp luminosity increased.
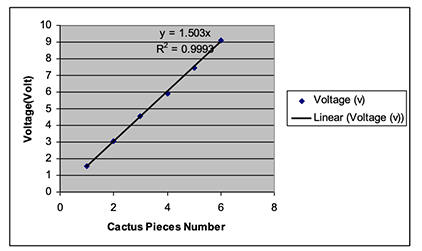
The result from Figure 3 shows that the R2 (Least Square Regression) fit is 0.9993, which indicates an excellent linear fit for the voltage versus number of cactus pieces. In addition, the data for the electrode set and the cactus electrolyte give a linear equation of: Y = 1.503X
After connecting four cactus electrochemical cells in series using Mg/Cu electrodes, the generated potential difference was enough to light the 5 mm white LED lamp. The potential differences average during the 15-min interval are presented in Table 3.
The potential differences average for the three trials was 6.15 V. To determine the importance of using Mg/Cu electrodes, the experiment was repeated by using 8 pieces of cactus as electrochemical cells connected in series with Zn/Cu electrodes. The results obtained are shown in Table 4.
Table 5 shows that the potential difference average for the three trials was 6.76 V. By comparing the potential difference average of 4 cactus electrochemical cells with Mg/Cu electrodes with 8 cactus electrochemical cells with Zn/Cu electrodes, it was clear that the potential difference average for the 8 cells was more than the potential difference average of 4 cells by only 0.27 V despite duplicating the number of pieces.
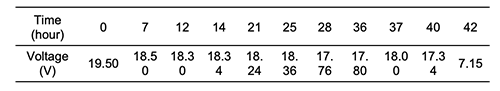
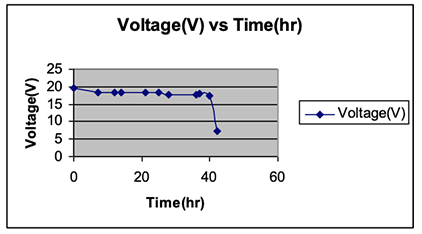
To study the time period of continuously lighting an LED lamp and potential difference stability, an experiment was carried out by connecting 13 pieces of cactus in series. The collected data, as presented in Table 5 and Figure 4, showed a semi-stable potential difference for more than 40 hours, unless the magnesium stripe broke down; then, consequently, the potential difference was decreased to 7.15 V.
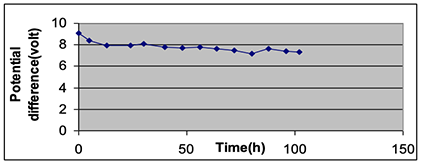
Comparing the potential differences over the 40-hour period showed a change in the potential difference at the end of the period by only 11%. The breakdown of the magnesium stripe decreased the potential difference to 7.15 V. This problem can be resolved by using a thicker electrode, hence the lighting time period will elongate. Figures 5 and 6 showed the correlation between potential difference and time for 6 pieces connected in series for a period of 102 hours.
This test was carried out to examine the potential difference and current stability after 40 hours, as is mentioned in Table 5, and to determine the longest period to keep the LED lamp lighting with good luminosity. The results showed that the potential difference decreased from 9.10 V at the beginning of the experiment to 7.35 after 102 hours by a decrement percentage of approximately 16%. A modified design of cactus battery was carried out as in Figure 7.
This design was able to generate electricity for a long time, more than two weeks (336 hours) with continuous LED lamp glow-up. The new design was carried out by increasing the thickness of the magnesium electrode and isolating the cutting cactus pieces into plastic bottles and connects it in series. Another well-modified cactus battery, as shown in Figure 8, can light up the LED lamp brightly.
This was built from the same electrodes by using cactus extract instead of cactus plates, as is explained in the procedure. The result of this application has promising future applications like emergency lighting, desktop lighting, etc. Table 6 shows the voltage and current variation for 6 cactus extract electrochemical cells connected in series.
Potential difference average during 6 hours of onetime soaking is 8.64 V, while the Conclusion The experiment proved that using Mg/Cu electrodes instead of Zn/Cu electrodes enhanced the generated potential difference by 50%. Moreover, using these electrodes decreased the number of cactus pieces needed to generate the same potential difference by 40%. Regarding the potential difference with time, the results proved the ability to produce a semi-stable high voltage for more than 4 days, where the LED lamp luminosity was still very good. The cut cactus pieces and cactus plate extract could be viable soon due to the low cost of the raw materials, easy applicability, as well as the long-time period of glow-up together with the high current values of cactus battery extract (1 mA). The cactus plate contains substances such as citric acid which under certain circumstances act as electrolytes. The electrons flow through wires from the anode to the cathode. This process generates electricity just the same way as a voltaic battery. References
|