|
Introduction Diabetes mellitus refers to a cluster of metabolic disorders characterised by a spike blood sugar or glucose (hyperglycaemia) affixed with a defect in insulin secretion, action, or both as well as aberrations in the intermediary metabolism of carbohydrates, proteins, and lipids [1-3]. In some cases, symptoms are absent or not severe, and afterward hyperglycaemia, which is enough to cause pathological and functional changes may emanate for a protracted period before the diagnosis is made [4]. The effects of diabetes embody long-term damage, dysfunction, and failure of different organs. It is often related to subsequent symptoms such as blurring of vision, excessive thirst (polydipsia), excessive feeding (polyphagia), excessive urination (polyuria), and weight loss [5]. Consequently, ketoacidosis reportedly develops as a result of complications resulting in stupor, coma, and in absence of effective treatment could result in death [6]. While two types of Diabetes mellitus are generally known, the predominant Type 2 is often managed using expensive conventional hypoglycaemic drugs, which include glibenclamide and metformin [5,6]. The disease is a menace which plagues people of all races and has a higher co-morbidity in regions where Western medicine is inaccessible or unaffordable. As a result of the unaffordability and side effects of current mainstream drugs, which include exacerbated renal and hepatocellular injury and disorder, diarrhoea, lactic acidosis, among others, several indigenous medicinal plants have been evaluated as a potential remedy for minimising these incidences or effects [2-5]. Medicinal plants have been reported to possess different pharmacological mechanisms in combating diabetes [4,7]. However, in modulating postprandial hyperglycaemia, reducing the rate of conversion of carbohydrates into glucose by inhibiting α-amylase, a carbohydrate hydrolysing enzyme, plays a vital role in the treatment, particularly in Type 2 diabetes [7-10]. Vernonia amyg-dalina of the family Asteraceae is one of the important medicinal plants used in the treatment of diabetes in folkloric medicine that has been proven to possess strong anti-inflammatory, antimalarial, antibacterial, antioxidant, and anticancer properties, amidst other scientifically proven pharmacological activities [11-15]. It is often referred to as bitter leaf on account of its bitter taste. It is a perennial shrub of an average height from 2-5 m, widely grown and consumed as a nutritional vegetable in Africa and Asia [16-18]. The plant has a rough bark with dense black straits, and elliptic leaves that are about 6 mm long. The leaves are green and have a characteristic odour with a bitter taste, which is due to the presence of phytocompounds such as alkaloids, saponins, glycosides, and tannins [11,16]. Δ7, 9(11) stigmastane-type steroid glycosides, such as vernonioside A1, vernonioside A2, vernonioside A3, vernonioside A4, vernonioside B1 and vernonioside B2, have been identified as the main constituents in the plant [19]. In this study, the bioactive principles in V. amygdalina root were isolated, characterised and subjected to in vitro alpha-amylase inhibition evaluation using 3,5-dinitrosalicylic acid alpha-amylase inhibitory assay. The isolated and characterised four compounds exhibited interesting in vitro alpha-amylase inhibition potential, worthy of further exploration.
Materials and Methods General 1H and 13C NMR spectra (400 MHz) were recorded on a JEOL 400 MHZ spectrometer operating at 400 (1H-NMR) and 100 (13C NMR) MHz at 298 K in DMSO-d6. Chemical shifts were reported in δ (ppm) values. The IR spectra were obtained on a Shimadzu 8400s spectrometer. Chemicals and Regents Pre-coated silica gel 60 F254 for thin-layer chromatography (TLC) and silica gel (60-200 mesh) for column chromatography (CC) were obtained from LOBA Chemie PVT, India. Alpha-amylase (A3306), glucose assay kit (glucose oxidase/peroxidase kit), soluble potato starch (33615), sodium phosphate monobasic (71496), dinitros- alicylic acid (609-99-4), acarbose and sodium phosphate dibasic (71640) were analytical standard. All solvents were analytical grades and redistilled before use where necessary. Plant Material Vernonia amygdalina root material were collected from the plantation of African Centre for Herbal Research Ilorin (ACHRI) University of Ilorin, Ilorin, Nigeria, and identified at the herbarium of the Department of Plant Biology, Faculty of Life Sciences, University of Ilorin, Ilorin, Nigeria where voucher specimen number UILH/001/972/ 2021 was assigned. The plant materials were air-dried, pulverized, and temporarily stored in a cool environment before further work. Extraction and Isolation The pulverized V. amygdalina (1.333 kg root) materials were subjected to successive sequential cold extraction starting with n-hexane, followed by ethyl acetate, then methanol, and finally water in a stoppered container for five days each. The extracts were concentrated at reduced temperature using a vacuum rotary evaporator and water bath as appropriate. The methanol crude root extract (12 g) obtained was subjected to elution on silica gel-packed column chromatography and eluted with solvents of increasing polarities using various solvents combinations comprising of hexane, ethyl acetate, methanol, and water to obtain eighty fractions. The fractions were examined on TLC and fractions with similar profiles were pooled together to afford seventeen combined fractions (A to O, S1, and S2). Fraction H (5 g) was rechromatographed on a silica gel packed column chromatography eluted with solvents of increasing polarities using solvents comprising of ethyl acetate, methanol, and water to obtain 50 sub-fractions (100 mL each) which were pooled together using TLC to obtain thirteen sub-fractions (Ha1-5, Hb-Hg, H4, and H5). Fraction H4 was further purified by washing with methanol, then freeze-dried to yield compound 1, vernoamyoside C. Fractions J, K, S2 were purified by washing with acetone to yield compounds 2, vernoniamyoside D, 3, luteolin-7-o-glucoside and 4, vernilorinoside, respectively. Purified isolated compounds were subjected to proton 1H nuclear magnetic resonance (NMR) and carbon 13C NMR, and Fourier-transform infrared spectroscopy (FTIR). The spectra obtained were used for the structural elucidation and characterization of the isolated compounds. DNSA Alpha-amylase Inhibitory Assay This assay was carried out using a standard procedure [20], based on measuring the oxidizing capacity of reducing sugars in a reaction with DNSA (3,5-dinitrosalicylic acid). A total of 250 μL of extract of varied concentration ranging from 50 to 400 μg mL-1 was placed in a tube and 250 μL of pancreatic α-amylase solution (0.5 mg mL-1) in 0.02 M sodium phosphate buffer (pH 6.9) was added. The mixture was incubated at 25°C for 10 mins, after which 250 μL of starch solution (1 %) in 0.02 M sodium phosphate buffer (pH 6.9) was added. This reaction mixture was again incubated at 25°C for 10 minutes. The reaction was finally quenched by 500 μL of 96 mM 3,5-dinitrosalicylic acid (DNSA) reagent, and further incubated in boiling water for 5 minutes and then cooled to room temperature. The content of each test tube was diluted with 5 mL distilled water and the absorbance was taken at 540 nm on a spectrophotometer. The absorbance of control was also measured as the assay was carried out in triplicate. The results were expressed as percentage inhibition of α-amylase activity using the following equation.
Statistical Analysis Data collected in replicate were analysed using GraphPad Prism 9.2.0 software. Results were expressed as mean ±SEM (Standard error of mean). Data were compared using Anova one way and P<0.05 was considered to indicate a statistically significant difference.
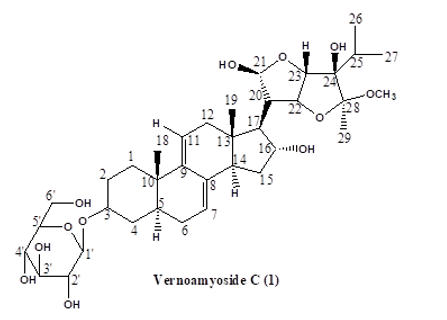
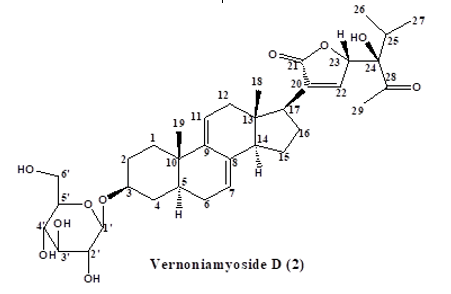
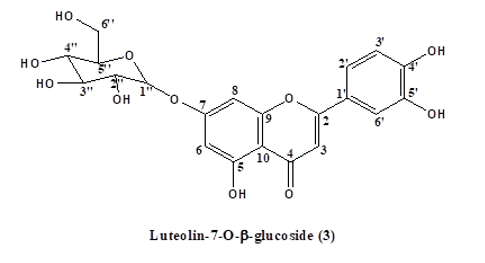
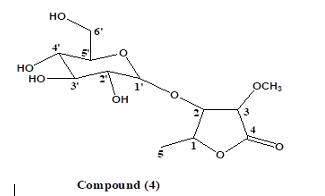
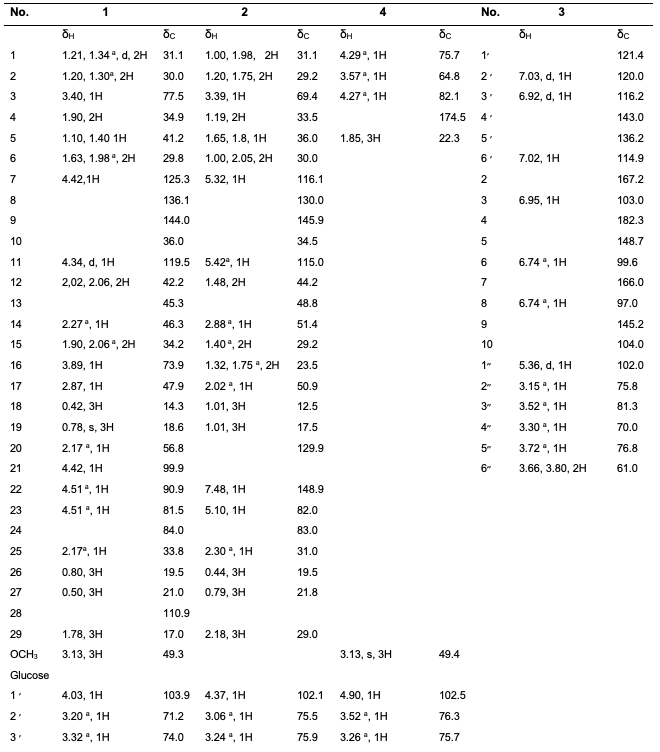
Results Characterization and Structural Elucidation of Bioactive Compounds The chromatographic elution afforded four compounds (Fig. 1).
The NMR characterisations are as depicted (Table 1). Compound 1: Vernoamyoside C was eluted with methanol-water (9:1) as a yellow powder. IR (KBr)Vmax(cm-1): 3404 (O-H stretch), 2934/2876 (sp3-CH stretch), 1632/1605, 1514 (C=C stretch), 1447 (CH2 bend), 1377 (CH3 bend), 1258/1163 (ester C(O)-C stretch), 1082/1051 (alcohol C-O stretch). The 1H and 13C NMR data (Table 1) indicated a methoxyl group at δH 3.13 that showed the peak from H-16 (δH 3.89) to Me- 18(δH 0.42) and H-20 (δH 2.17) suggested that the additional OH-16 has an α-configuration. The NMR data was compared with literature data [19]. Compound 2: Vernoniamyoside D was obtained as a yellow powder from the Si-gel CC eluted with EtOAC: MeOH (3:2).IR (KBr)Vmax(cm-1): 3404 (OH), 2936/2878 (sp3-CH stretch), 1771 (conjugated lactone C=O stretch), 1705 (C=O stretch), 1636/1609, 1516 (C=C stretch), 1447 (CH2 bend), 1377 (CH3 bend), 1261/1142 (ester C(O)-C stretch), 1078/1055 (alcohol C-O stretch). The 13C NMR data indicated 35 carbon signals, and also comparing both 1H and 13C NMR data (Table 3.3) shows compound 2 to be vernoniamyoside D. The result is in tandem with literature [21]. Compound 3: Luteolin-7-O-β-glucoside was eluted as a yellow powder from the Si-gel CC with EtOAC: MeOH (5.5:4.5).IR (KBr)Vmax(cm-1): 3397 (OH), 2934/2878 (sp3-CH stretch), 1707 (C=O stretch), 1630/1605, 1518 (C=C stretch), 1447 (CH2 bend), 1375 (CH3 bend), 1267/1159 (ester C(O)-C stretch), 1078/1055 (alcohol C-O stretch). Through the analysis of the NMR data (Table 1) and spectra comparison of a compound in Boudoukha et al. (2018) literature [22], the isolated compound was identified as luteolin-7-β-glucoside. Compound 4: Vernilorinoside obtained via the elution on Si-gel CC produced a yellow powder (17.66 mg). IR (KBr)Vmax(cm-1): 3258 (OH), 1636 (C=O stretch), 1409 (CH3 bend), 1264 (ester C(O)-C stretch), 1059 (alcohol C-O stretch).
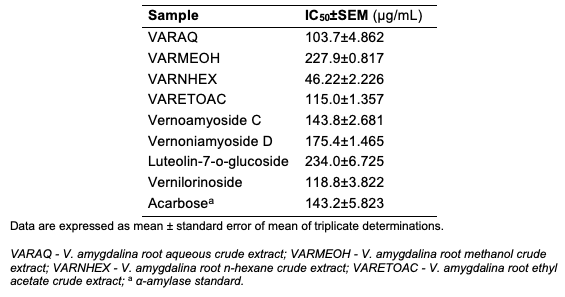
Results of the Alpha-amylase Assay The result of the alpha-amylase inhibition potential is as presented (Table 2).
Discussion The results of α-amylase inhibitory activity showed that all the crude extracts inhibited amylase activity (Table 2). The n-hexane crude extract exhibited the highest inhibitory activity (IC50 = 46.22±2.226 μg/mL) suggesting an improved activity compared to other crude extracts, the standard, acarbose with IC50 = 143.2±5.823 μg/mL) against α-amylase and methanol extract (IC50 =227.9±0.817 μg/mL) showed the least in- hibitory activity. Similarly, isolated bioactive compounds 1-4 showed varied IC50 values (Table 2). With reference to the standard, data obtained were not significantly different at P<0.05. Compound 4, vernilorinoside, a new compound with IC50 =118.8±3.822 μg/mL), exhibited the highest inhibitory activity while compound 3 (IC50 =234.0±6.725 μg/mL) exhibited the least inhibitory activity. Although the mechanism of action of the compounds has not been studied, the small size of the Compound 4, importantly the aglycone unit may enhance the potency of the compound. All samples including the crude extracts and bioactives showed a concentration-dependent α-amylase inhibitory activity. In order to corroborate the results obtained in the studies, the evaluation of the isolated compounds in animal model is recommended. Conclusion In this study, four compounds (1-4) were isolated and purified from the root of V. amygdalina and characterized using spectra data obtained. To the best of our knowledge, this is the first report confirming the presence of compounds 1, 2, 3, and 4 in the root of the plant. The analysis of the results showed that the crude extracts and isolated phyto-compounds from V. amygdalina root are potent α-amylase inhibitors. It further suggests that V. amygdalina holds promise in the overall treatment or management of Type 2 diabetes by decreasing postprandial hyperglycaemia through the inhibition of the α-amylase enzymatic pathway. The crude extracts and the isolated compounds subjected to DNSA assay suggest the possibility of the amelioration of Type 2 diabetes disease via mechanisms that may be evaluated in the future. Compounds 1-4 which are glycosides may be further studied as a possible biomarker of the plant in addition to the pharmacological potentials exhibited. Future studies should be directed to exploring the in vivo potentials of the compounds. Acknowledgments Authors acknowledge the plant material donation from African Centre for Herbal Research, Ilorin (ACHRI), University of Ilorin, Nigeria.
Conflict of Interest The authors declare no conflict of interests.
References
|
|||||||||||||||||||||||||||||