|
Introduction Pathogenic bacteria and fungi have emerged over time in our environment, posing a serious danger to public wellbeing [1]. Bacteria such as Shiga toxin-producing Escherichia coli, Salmonella enterica, Listeria monocytogenes and fungal pathogens such as Aspergillus niger and Aspergillus flavus have been linked to more serious illnesses, complications, and even deaths [2,3]. They are the major causes of acute health problems such as diarrhea, vomiting, nausea, stomach cramps, migraines, paralysis, and paresthesia [4]. Treatment of these infections has resulted in an increased economic burden on diverse parts of the world, especially in developing nations [2]. Despite the abundance of antimicrobial med-ications for treating illnesses resulting from bacterial and fungal infections, there are growing inefficacies in these treatment options due to the development of resistance caused by horizontal gene transfer as well as unregulated usage of antimicrobial drugs [5]. This has necessitated the need to improve or introduce a novel therapeutic regimen that could replace conventional drugs [6]. Recently, metal-ligand adducts have become of critical importance in the development of medication due to their therapeutic efficacy, which have been the subject of numerous studies [7,8]. The addition of metal ions to biologically active ligands has been shown in the literature to primarily improve their properties [6]. Several metal-ligand medications that primarily comprise pyrans, thiazole, imidazole, piperidines and other sulfur-, nitrogen-, or oxygen-containing heterocyclic compounds have recently been developed [9,10]. Nitrogen donor ligands, particularly imidazole derivatives, have shown to be appealing tools for coordinating metal ions in several ways. The electron-rich imidazole heterocyclics are able to build complexes with specific biological and redox functions due to their good donor properties [11]. Carboxylate ligands, such as 4-methyl-benzoic acid, have also been reported in many studies such as coordination chemistry, materials chemistry, and supramolecular chemistry owing to their capacity to establish stable bonds with metal ions and display diverse coordination modes [12,13].
In the present study, Zn (II) and Cu (II) complexes were synthesized using 4-methy-lbenzoic acid (Figure 1a) and a nitrogen donor heterocyclic ligand 2-methylimidazole (Figure 1b), they were structurally elucidated and their antimicrobial potentials against pathogenic organisms were investigated with both experimental and in-silico molecular docking studies.
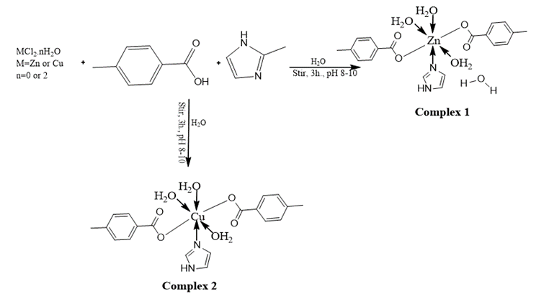
Materials and Methods All the reagents used were of analytical grade. The ligands were obtained from Sigma Aldrich, while the salts and solvents were obtained commercially. The compounds' IR spectra in KBr pellets were studied with a SHIMADZU FTIR (FTIR 8400s) in the frequency range of 400-4000 cm-1. The complexes' molar conductance (1 x 10-3 molL-1) was determined in DMSO at 25oC using a HANNA conductivity meter with a cell constant of 0.83. The elemental analysis and melting point were performed on a CHNS/O Perkin Elmer Analyser (2400 series II) and MPA100 Optimelt Automated Melting Point System (SRS), respectively. The GCMS analysis was conducted by on an Agilent-Technologies 6890N Network GC system, equipped with an Agilent-Technologies 5975 inert XL Mass selective detector, located in Little Falls, CA, USA. The PXRD patterns were obtained using an Empyrean diffractometer in the angle range 2θ = 0 – 70o with Cu-Kα1(λ = 1.5406) and Cu-Kα2(λ = 1.5444). Synthesis of [Zn(4MBA)2(2MIm)(H2O)3].H2O (1) Complex 1 was prepared, as described by Chen et al. [14] by dissolving 1.0 mmol ZnCl2 (136.3 mg), 1.0 mmol 4MBA (136.1 mg) and 1.0 mmol 2Mim (82.1 mg) in 10 mL distilled water. The mixture's pH was brought to a range of 8 to 10 with the addition of 0.5M KOH and stirred continuously for 3 hrs at room temperature. The resulting white pre-cipitate was filtered, washed with distilled water, and then dried using anhydrous calcium chloride in a desiccator. Analytical data for Complex 1: White powder, Yield: 67 %, m.pt.= 248oC, Anal. Calcd. for C20H28N2O8Zn, (MW = 489.8 g/mol) %:C; 49.04, H; 5.76, N; 5.72, found C; 49.06, H; 5.73, N; 5.70.Synthesis of [Cu(4MBA)2(2MIm)(H2O)3] (2) Complex 2 was prepared following the same procedure as complex 1, with CuCl2.2H2O (1.0 mmol; 170.5 mg) as the metal salt. The resulting blue precipitate was filtered, followed by a thorough wash with distilled water. It was subsequently dried using anhydrous calcium chloride in a desiccator. Analytical data for Complex 2: Purple powder, Yield: 62 %, m.pt.= 235oC, Anal. Calcd. for C20H26CuN2O7, (MW = 467.9 g/mol) %:C; 51.11, H; 5.58, N; 5.96, found C; 51.10, H; 5.59, N; 5.90. The synthetic route to complexes 1 and 2 is represented in Fig. 2.
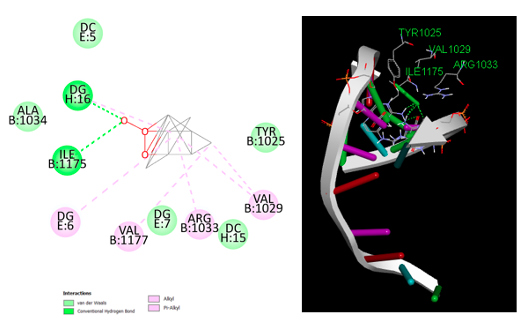
Antimicrobial Studies The assessment of antimicrobial properties for both the ligands and the newly synthesized compounds was conducted in accordance with the procedure outlined by Obaleye et al. [15]. The sensitivity of bacterial isolates, including P. aeruginosa, S. aureus and E. Coli and strains of fungi viz: A. niger, Candida spp. and A. flavus, was investigated using the agar well diffusion method. The Muller-Hinton nutritional agar plates were carried out in duplicates per test organisms. Test organisms were made to equivalent 0.5 McFarland standards and inoculated in sterile petri dish. Sterile cork borer was used to make five 10 mm equivalent holes on the surface of the MHA agar. The test compounds (6 mg/mL) each was introduced into four of the holes while the control at the center hole contained DMSO. The bacterial and fungal strains were incubated for 24 h and 48 h at 37°C, respectively. The zone of inhibition’s diameter was carefully assessed and recorded in millimeters (mm). All inhibition zones with a diameter of less than 10 mm are deemed inactive against a given strain. Molecular Docking The molecular interaction and binding affinity of the synthesized compounds were examined at a resolution of 3.34 using Staphylococcus aureus DNA gyrase (PDB ID: 2XCT) retrieved from the Protein Data Bank. According to the AutoDock Vina 4.2 protocol, the protein was processed by removing the water molecules and adding polar hydrogen and cofactors. The grid box was centered on vital amino acid residues surrounding the protein macromolecule's active sites (Tyr1025, Val1029, Arg1033, Val1177, Ile1175, Ala1034). AutoDock Vina was used to analyze the complexes with the target receptor.
Results and Discussion The molar conductivities of complexes 1 and 2 are presented in Table 1. The conductivity values, 15.35 for complex 1 and 14.57 for complex 2, were less than 40 Scm2mol-1. This suggests that the complexes are non-electrolyte in DMSO solvent [16]. Moreover, the results above indicate that there are no counter ions outside the coordination sphere of the two complexes [17].
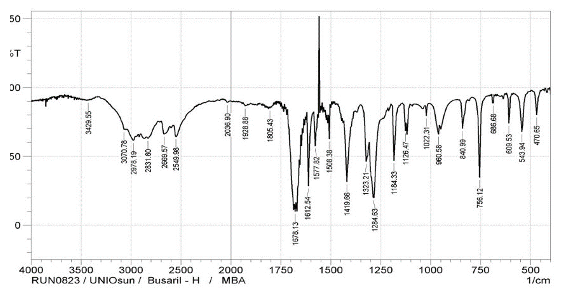
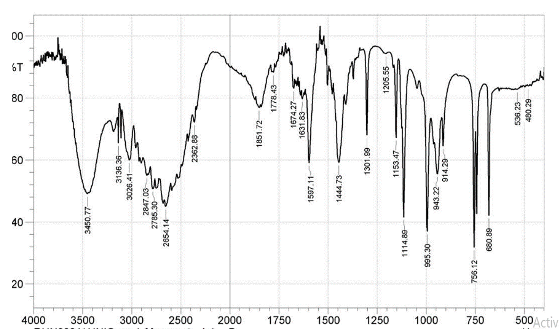
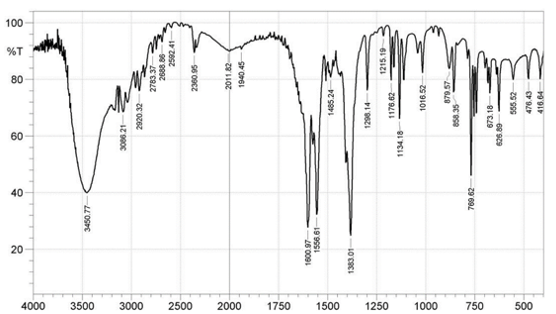
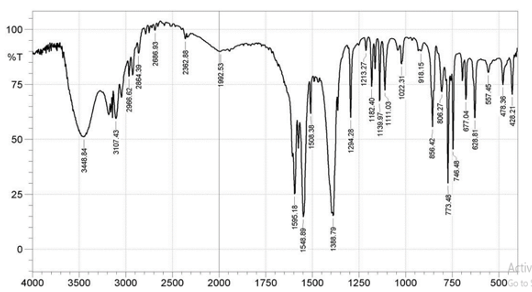
Infrared Spectra The FTIR spectra of the ligands and complexes are presented in Fig. 3a-d, where some notable vibrational bands are observed.
At 1678 cm-1, the carbonyl band of 4MBA ν(C=O) was observed, while at 1284cm-1, an asymmetric aromatic stretching band ν(CO) was observed (F ig. 3a) [16,18]. The peaks at 1660cm-1 and 1383cm-1 for complex 1 (Fig. 3c); 1595cm-1 and 1388cm-1 for complex 2 (Fig. 3d) are attributed to νasym(COO-) and νsym(COO-), respectively. The difference ∆v = [Vasym(COO-) - Vasym(COO-)]; 217cm-1 for complex 1 and 207cm-1 for complex 2 reflects a monodentate mode of coordination of the carboxylate group. The sharp peaks at 1556cm-1 (Fig. 3c) and 1548cm-1 (Fig. 3d) in the complexes 1 and 2, respectively, are attributed to aromatic C=C bond of the benzoic acid while the coordinated water molecules are found by the bands at 626cm-1 and 628cm-1, respectively, and these values agree with the literature reports [19]. In the spectrum of free 2MIm (Fig. 3b), there is no significant shift in the NH band (3450cm-1) when compared with that of complex 1 (3450cm-1) and complex 2 (3448cm-1) to suggest coordination but indicates the involvement of 2MIm in coordination.
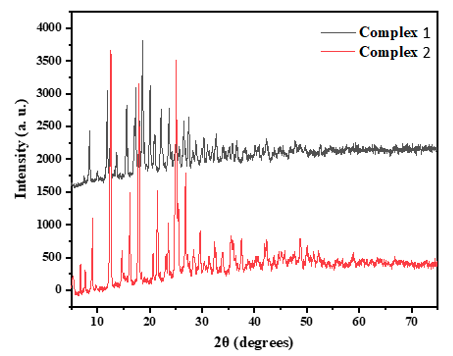
XRD Pattern The powder XRD patterns of complexes 1 and 2 are shown in Fig. 4. The difference in their XRD pattern is an indication of their different structures. The Zn ion in complex 1 exists in cubic system matching JCPDS card number 00-412-2713 with space group P 21 3 having cell parameter a= 20.1619 Å. The Cu ion in complex 2 exists in orthorhombic system matching JCPDS card number 00-430-5279 with space group Pbcn. The cell parameters are a= 8.1953Å, b=7.5577Å, c= 13.4593Å. The X-ray diffraction (XRD) pat-terns of the newly synthesized Zn (II) and Cu (II) compounds exhibited distinct crystalline structures, as evidenced by the pronounced peaks in their XRD patterns.
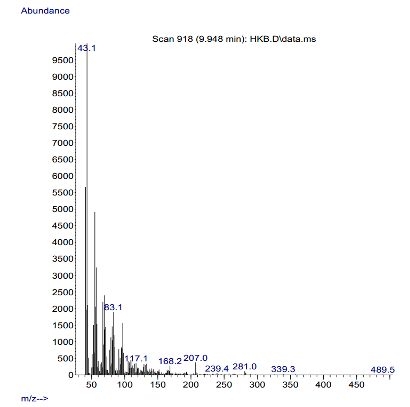
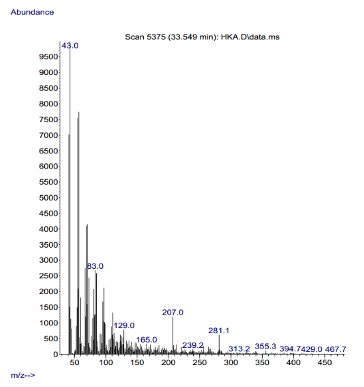
Mass Spectra The mass spectra of the complexes are shown in Figures 5a and 5b.
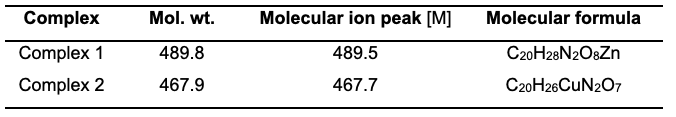
The observed molecular ion (M) peaks were used to confirm the proposed molecular weights of the complexes and hence determine the molecular formula. Complexes 1 and 2 show a molecular ion peak (m/z) at 489.5 and 467.7, respectively, as shown in their respective spectra. These peaks are consistent with the proposed molecular weights of the respective metal complexes as shown in Table 2. These findings agree with the hitherto studies of Damena et al. [20] and Uddin et al. [21].
Antimicrobial Studies
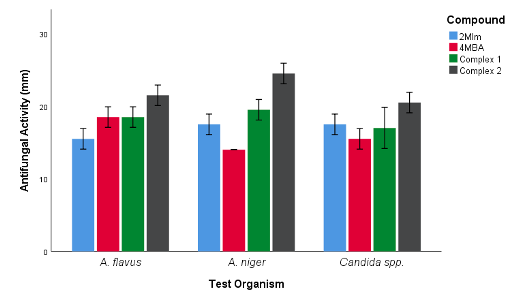
Also, amongst the compounds tested for antifungal activity, complex 2 was observed to be the most effective in inhibiting the growth of A. flavus, A. niger and Candida spp. Similar antifungal activities was observed for complex 1 and 4MBA ligand against A. flavus and complex 1 and 2Mlm against Candida spp. (Figure 6b).
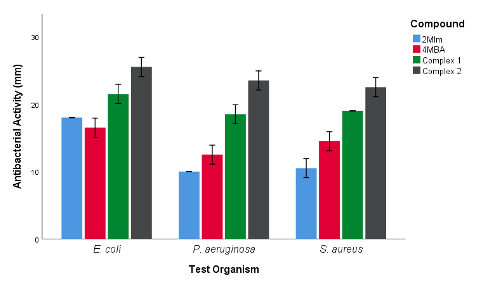
Against the tested organisms, complexes 1 and 2 showed differential antimicrobial activities compared to the free ligands. Notably, complexes 1 [Zn(4MBA)2(2MIm) (H2O)3].H2O and 2 [Cu(4MBA)2(2MIm) (H2O)3] had higher antibacterial activity than 4MBA and 2MIm. In this present study, complex 2 displayed a high antibacterial activity when compared to the free ligands (4MBA and 2MIm) and complex 1. This is consistent with report of Kaushal et al. [22] that reported the excellent antibacterial activity of copper complex against S. aureus. Similarly, Oladipo et al. [23] and Krishnegowda et al. [24] have described the excellent antibacterial activity of complex of copper against P. aeruginosa, E. coli and S. aureus. Also, complex 1 dis-played a remarkable antimicrobial property against gram-negative E. coli and gram-positive P. aeruginosa and S. aureus. This is in line with reports of Ali et al. [25]; Boughougal et al. [26] and Basu Baul et al. [27] that described the antimicrobial potency of the zinc complex against multiple drug resistance pathogens. Overall, chelation raised the liposolubility of the complexes, which was boosted due to π electrons delocalization over the chelate ring of the complex, which increased the antibacterial activity of the synthesized metal complexes against the tested strains of organisms [12,28,29].
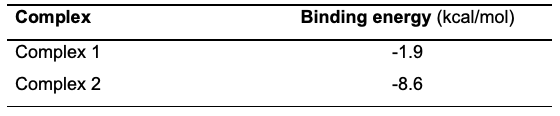
Molecular Docking Studies The metal complexes were tested for their ability to inhibit S. aureus DNA gyrase. The complexes' orientation to the enzyme's active regions was determined by employing automated docking. Table 3 shows the binding energy for the interaction, while Figure 7 shows the complex with the best binding affinity to the active sites on the DNA gyrase.
Conclusion The two complexes, (1) [Zn(4MBA)2(2MIm) (H3O)3].H2O and (2) [Cu(4MBA)2(2MIm) (H3O)3], have been synthesized, characterized, and studied for antimicrobial activity . The FTIR spectra revealed that the 4-methylbenzoic acid ligand coordinated through the carbonyl oxygen atom of the carboxylate group in a monodentate mode in both complexes. In addition, the 2-methyl imidazole ligand coordinated in a monodentate mode through the imidazole nitrogen atom. The two complexes were proposed to exist in an octahedral coordinate system based on the comparison of the results from the elemental analysis, FTIR and mass spectra. Complex 1 was cubic and had the space group P 21 3, whereas complex 2 had the space group Pbcn and was orthorhombic. The two complexes had strong antibacterial action against the selected antimicrobial organisms; however, complex 2 displayed a better inhibitory tendency. This is in agreement with the results of the molecular simulation which showed that complex 2 had the higher binding energy against DNA gyrase and thus, the better antimicrobial potential. Therefore, complex 2 could then be considered as a preferred antimicrobial agent in the future.
Conflict of Interest The authors declare no conflict of interest.
References
|
||||||||||||||||||||||||||||||||||||||||||||||||