|
|
|
|
| The Chemist Volume 94 | Number 2 |
 
|
| |
Phytochemistry and Pharmacology of Anogeissus leiocarpus (DC.) Guill. & Perr. - A Review
|
| |
Abstract: The relevance of medicinal plants as a primary source of therapeutic agents in the modern world cannot be overemphasized. Anogeissus leiocarpus (A. leiocarpus),of the family Combretaceae, is a renowned medicinal plant used in folkloric medicine for the management of various illnesses, particularly in developing countries. In African traditional medicine, the edible stem bark used as a chewing stick reportedly possesses numerous biological activities. Stem bark extract from A. leiocarpus exhibits anti-parasitic, anti-hypertensive, and anti-tubercular effects. Additionally, A. leiocarpus bark extract is used in traditional medicine in Sudan to alleviate cough and in Ivory Coast to treat parasitic diseases. The plant reportedly possesses other medicinal properties, including anti-diabetic, anti-inflammatory, anti-malarial, and anti-cancer activities due to the presence of important phytochemicals, such as phenols, flavonoids, saponins, and alkaloids. Compounds including, serinic acid, arjungenin, isoquercetin, vitexin, kaempferol and some others have been identified as bioactives from various parts of the plant.
Key Words: Anogeissus leiocarpus, secondary metabolites, anti-malarial, antioxidant, anti-cancer |
|
|
Introduction
Indigenous plants are a primary source of secondary metabolites, which play an intriguing role in traditional medicine [1]. The presence of several bioactive chemicals with chemo-preventive, antioxidant, antifungal, anti-inflammatory, antibacterial, analgesic, and other activities is what gives these plants their medical relevance [2].
Africa and other developing nations still rely heavily on medicinal plants as a therapeutic option for the management of variety of illnesses and diseases [3]. Recently, more attention has been directed at finding new drugs from a plant origin. Hence, there is a compelling need to find new bioactive compounds and new “leads” with vital pharmacological activities from herbal plants, such as the Anogeissus leiocarpus, a tropical indigenous medicinal plant in Africa. A. leiocarpus is the most abundant tree species in the woodland [4], which can be used in the production of charcoal [5]. A. leiocarpus is consumed as herbal tea and used for therapeutic purposes in the treatment of many ailments [6]. The leaf of A. leiocarpus has been found to be effective in treating sickle cell anemia [7]. The stem bark and leaf of A. leiocarpus offers potential alternatives and conveniently accessible sources of antibacterial compounds for the treatment of numerous bacterially induced illnesses [8-10]. The stem bark extract of A. leiocarpus has been utilised for a long time in conventional tanneries as a native agent for softening hide and skin [11].
Methodology
Data were gathered from various online databases such as ScienceDirect, PubMed, Scopus, Google Scholar and Web of Science by selecting the most comprehensive, recent and relevant articles on Anogeissus leiocarpus from the year 2014 to 2023
Plant Profile
A. leiocarpus, a deciduous tree (Figure 1) native to Asia and Africa belonging to the Combretaceae family, flourishes in a variety of environments, including forests, savannas, bushlands, semiarid grasslands, and drylands [12-14]. A. leiocarpus, also called African birch or axle wood, is called Ayin in the South-West region of Nigeria.
The A. leiocarpus are endemic in the forests and savanna zones of the Sudanese region. Its extensive biological activities extend from the edge of the Sahara to the uppermost layer of wet tropical forests. Senegal to Cameroon in West Africa, as well as Ethiopia and East Africa, are other places where it can be found or grown. in they thrive well at both dry forests and at the riverbank of wet regions [15].
A. leiocarpus is a deciduous tree that typically reaches heights of 15 to 18 m and has light green foliage. In form, leaves range from elliptic to ovate-lanceolate, alternating to subopposite, and are 2 to 8 cm long by 1.5 to 3.5 cm wide [16]. The bark is fibrous with tiny scales, grey to beige in colour, and becomes blackish with age. The stems are coarsely pubescent. There are around 40 seeds of 10 g each that are spread by wind in an A. leiocarpus [16]. The leaves are attenuated at the base, pointed at the apex, and hairy below. The flowers lack petals and are bisexual ; two-centimetre-wide, yellow inflorescence globose heads. The fruits are globose cone-like heads that are extensively winged, dark grey, and 3 cm in diameter. It reproduces both vegetatively and by seeds [12].
A. leiocarpus has a long history of being used as an infusion to treat a number of diseases. Extracts from the roots, leaves, stem bark, and twigs are used to treat illnesses, such as gonorrhoea, wounds, acute respiratory tract infections, stomach infections, TB, dysentery, and malaria. The stem bark, which is typically consumed as chewing sticks or used as home beverages, is known to contain wound-healing, anti-pneumonia, anti-arthritis, antibacterial, anti-malaria and anti-trypanosomal effects [2,12,17-19]. Crude extract from this plant has been investigated to be effective in termite control [20]. Stem bark extracts have also been demonstrated to have the ability to protect liver function [21] and act as anti-parasitic, anti-hypertensive and anti-tuberculosis agents [22-25]. The aqueous extract of A. leiocarpus could be utilised as an alternate treatment and control method for coccidiosis [26].
Traditional Sudanese medicine uses a decoction of the bark to treat coughs [27]. The herb is used by traditional healers in the Ivory Coast to cure parasitic illnesses such malaria, trypanosomiasis, helminthiasis, and diarrhoea [28]. In traditional Togolese medicine, the decoction of the leaves is used to cure stomach problems and fungi infections including dermatitis and mycosis [29]. The plant extracts are effective in treating diabetes, ulcers, generalised body aches, blood clots, asthma, coughing, and tuberculosis [30].
Phytochemical Profile
Many potent phytochemical components found in A. leiocarpus have been demonstrated to be responsible for the therapeutic properties of the plant [31-34]. Secondary metabolites found in A. leiocarpus stems include alkaloids, tannins, flavonoids, cardiac glycosides, and saponins [35, 36]. Preliminary phytochemical screening of the Anogeissus leiocarpus stem bark for the major secondary constituents revealed that the plant, which was obtained from a local farm in Jigawa, Nigeria, was abundant in tannins and contained significant amounts of flavonoids, terpenes, and saponins, but was devoid of anthraquinones [12]. According to Hussaini et al. [37], the stem bark extract contained saponins, tannins, phenols, phytosterols but was devoid of flavonoids.
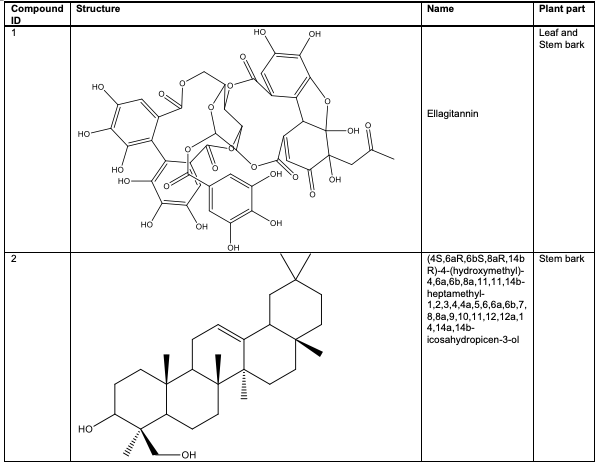
Despite its widespread use, only a few studies have established the phytochemical profile of A. leiocarpus stem bark to date [23,38]. In a qualitative chemical assessment of A. leiocarpus leaf and stem bark extracts by HPLC-ESI-MSn analysis, a significant number of phenolic components, including ellagitannins 1, and some flavonoids were identified [38].
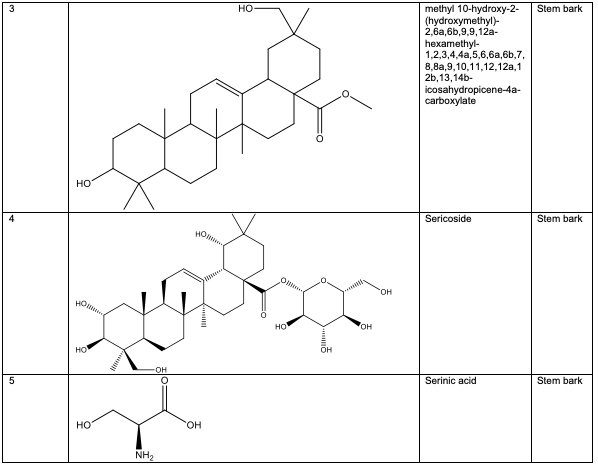
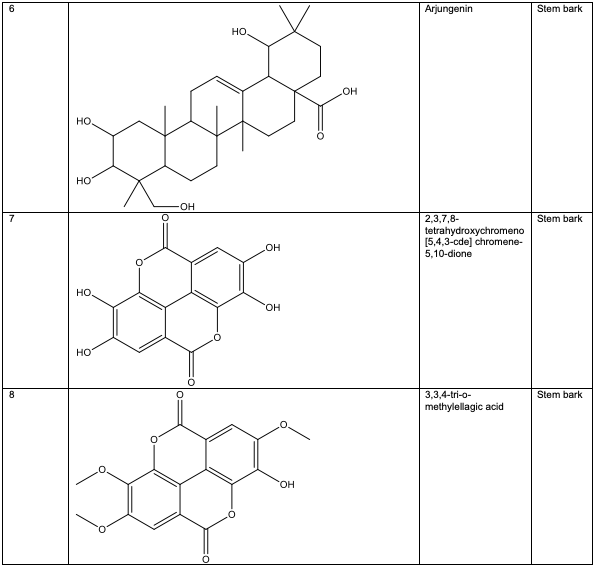
The stem bark of A. leiocarpus contains two oleanane-type compounds (4S, 6aR, 6bS, 8aR,14bR)-4-(hydroxymethyl)-4, 6a, 6b, 8a, 11,11,14b-heptamethyl-1, 2, 3, 4, 4a, 5, 6, 6a, 6b, 7, 8, 8a, 9, 10, 11, 12, 12a, 14, 14a, 14b-icosa-hydropicen-3-ol 2 and methyl 10-hydroxy-2-(hydroxymethyl)-2, 6a, 6b, 9, 9, 12a - hexa-methyl-1, 2, 3, 4, 4a, 5, 6, 6a, 6b, 7, 8, 8a, 9, 10, 11, 12, 12a, 12b, 13, 14b-icosahydropicene-4a-carboxylate 3, as well as other triter penoids, including sericoside 4, serinic acid 5 and arjungenin 6 [39,40]. Several ellagic acid compounds, including 2, 3, 7, 8-tetrahydroxychromeno[5, 4, 3-cde] chromene-5, 10-dione 7, were identified [23].
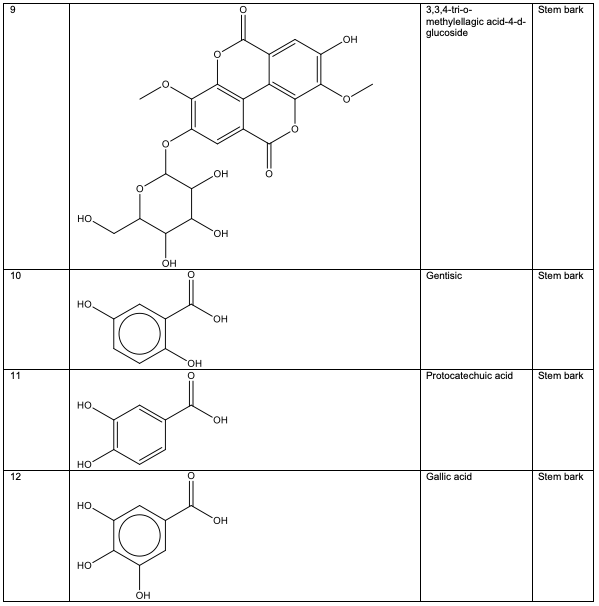
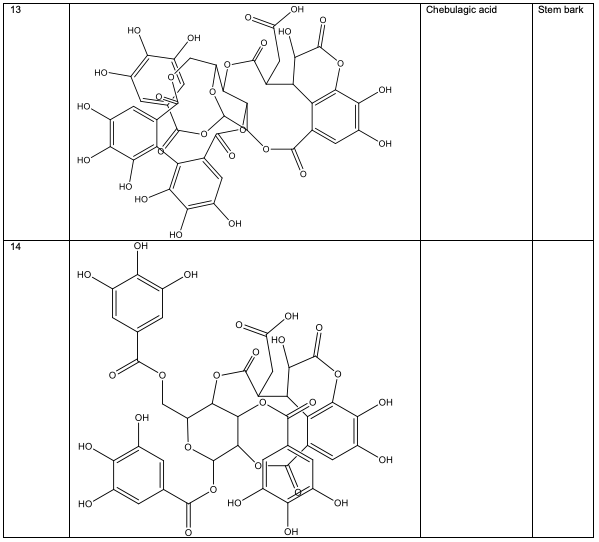
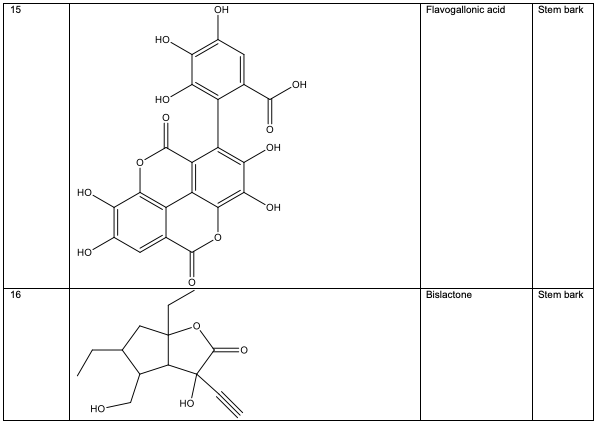
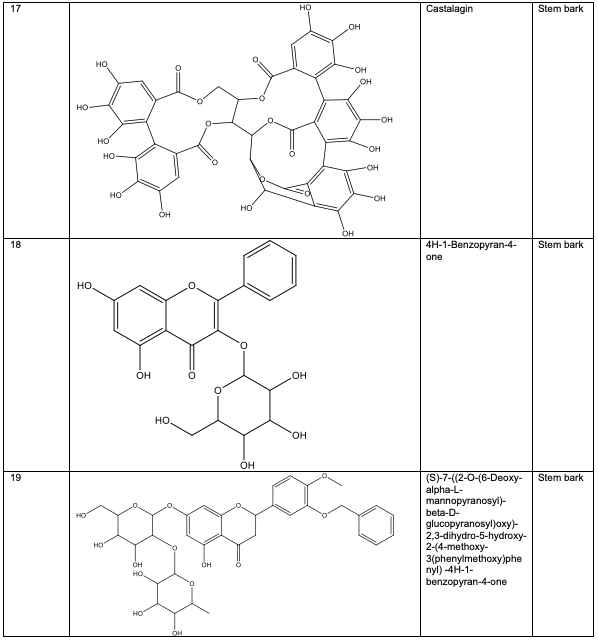
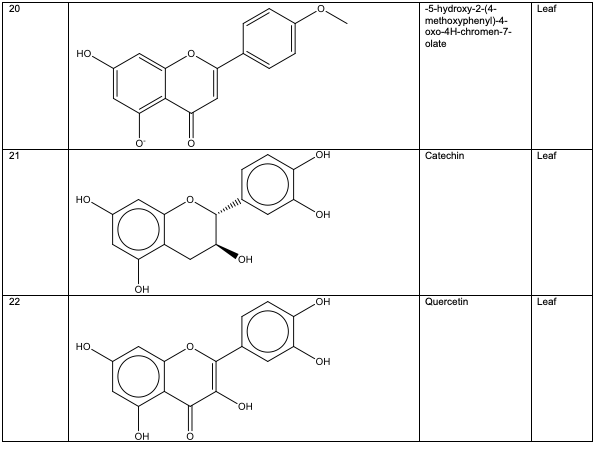
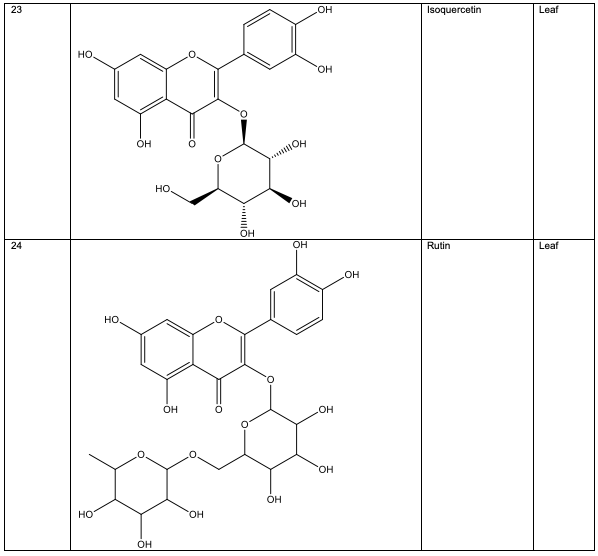
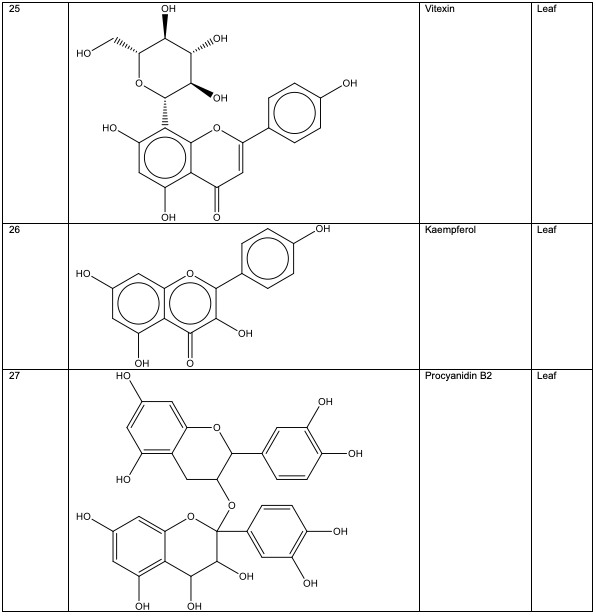
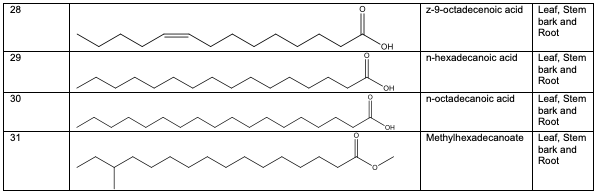
Polyphenolic substances found in the stem bark included 3, 3, 4-tri-o-methylellagic acid 8, 3, 3, 4 - tri-o-methylellagic acid – 4 - d-glucoside 9, gentisic 10, protocatechuic acid 11, gallic acid 12, chebulagic acid 13 and chebulinic acid 14. The stem bark also contained flavogallonic acid 15, bislactone 16, castalagin 17, and ellagic acid 7, [12]. 4H-1-Benzopyran-4-one 18, and (S)-7-((2-O-(6-Deoxy-alpha-L-mannopyranosyl)-beta-D-glucopyranosyl)oxy)-2, 3-dihydro-5-hydroxy-2-(4-methoxy-3(phenylmethoxy)phenyl) -4H – 1 - benzo-pyran-4-one 19. The leaf contained -5-hydroxy-2-(4-methoxyphenyl)-4-oxo-4H-chro-men-7-olate 20, catechin 21, quercetin 22, isoquercetin 23, rutin 24, vitexin 25, kaempferol 26, and procyanidin B2 27 [12]. Analysed essential oils with the aid of GC-MS obtained by hydro-distillation using a Clevenger-type apparatus from the leaf, stem bark and root of A. leiocarpus revealed the prominence of z-9-octadecenoic acid 28, n-hexadecanoic acid 29, n-octadecanoic acid 30 and methylhexadecanoate 31 [13].
Nutritional Values
The proximate analysis of the leaf of A. leiocarpus revealed a high content of crude protein (17.31%). The mineral analysis showed high levels of calcium and potassium, moderate levels of magnesium, iron and zinc, and low levels of copper and manganese [41]. Sawdust from A. leiocarpus (Hardwood) is more beneficial for growing mushrooms with a good nutritional composition that can promote good health in man [42].
Pharmacological Activities
A. leiocarpus has been subjected to a variety of in vivo and in vitro biological evaluations. This plant is equally known as a source of antimicrobial agents and for treatments of a variety of infection-related ailments [43]. Some of the pharmacological evaluations of the plant are as highlighted.
The aerial plant extract and supernatant of A. leiocarpus root bark significantly reduced serum and hepatic triglyceride levels, the amount of VLDL (Very Low-Density Lipoprotein) cholesterol and hyperlipidemic levels in mice. The crude extract and constituent fractions showed significant overall antioxidant activity [44]. It was discovered that A. leiocarpus crude extract and fractions possessed strong antioxidant [45] and anti-hyperlipidemic properties. The polyphenolic-rich extract of the plant may be useful in treatment of Diabetes mellitus [46]. A. leiocarpus leaves and stem bark extracts were similarly found to inhibit glucosidase activity [47]. The extract and the supernatant fraction of the roots of A. leiocarpus demonstrated a strong antidiabetic potential by hy-perglycemia reduction, hyperlipidemia and glucose intolerance in rats induced with diabetes [48,49]. In cases when insulin is unaffordable, A. leiocarpus can be used as an unconventional treatment for diabetes-related oxidative stress [50,51]. In diabetic patients, a crude ethanol extract of A. leiocarpus stem bark lowers blood glucose levels [52,53].
The antimicrobial properties of A. leiocarpus support the beliefs of traditional healers that the plant's roots and stem bark can treat a variety of diseases [54,55]. Various antimicrobial activities which include antibacterial and anti fungal activities of the plant have been established as highlighted.
The root of the plant reportedly possesses huge antibacterial potential against pathogenic organisms which include Escherichia coli. The root material is sold in Nigeria as chewing sticks for the prevention of oral infections and mouth odour. The study suggested that plants with huge antibacterial potential against oral germs could also possess extended activities against throat infections, gum disease, and tooth decay [56,57]. In another evaluation, the tested isolates exhibited resistance to the crude leaf extract of A. leiocarpus [58,59]. The in vitro susceptibility of five bacteria, including Staphylococcus aureus, Escherichia coli, Klebsiella aerogens, Pseudomonas aeruginosa, and Salmonella typhi to the leaf, bark, and root extracts of A. leiocarpus revealed the strong antibacterial properties of the extracts [60,61].
A plant source of anti fungal activity is A. leiocarpus [62-64]. The in vitro antifungal activity of root extracts from Anogeissus leiocarpus against Aspergillus niger, Aspergillus fumigatus, Penicillium species, Microsporum audouinii, and Trichophyton rubrum was investigated using the radial growth technique. The extracts inhibited the growth of all the test organisms significantly. The minimum inhibitory concentrations (MIC) and minimum fungicidal concen-trations (MFC) of the extracts ranged from 0.03 to 0.07 g/mL and 0.04 to 0.08 g/mL, respectively. A. leiocarpus appears to be effective as an antifungal drug [65]. The in vitro susceptibility of two fungi, Candida albicans and Aspergillus niger, to the leaf, bark, and root extracts of A. leiocarpus revealed the strong anti fungal properties of the extracts [60].
A. leiocarpus stem bark fractions and crude methanol extracts were found to be highly effective against a field isolate of Plasmodium falciparum. The study therefore validated the traditional use of this herb as an effective malaria treatment option [66]. The methanolic extract of A. leiocarpus has been considered locally to have the same antimalarial activities as artemisinin derivatives in malaria-infected organisms [67,68].
The aqueous extract of A. leiocarpus leaves exhibited antidiarrheal properties by delaying intestinal peristalsis and decreasing gastrointestinal output of fluids and electrolytes. This explains why this plant is used to treat diarrhoea in conventional medicine [69,70].
A possible source of anticancer through the angiogenesis pathway is A. leiocarpus [71]. According to Olugbami et al. [72], extracts from the leaves and roots of A. leiocarpus can inhibit the rapid replication of cancer cells. Ehrlich ascites carcinoma cell lines were prevented from proliferating by the root extract of A. leiocarpus, whilst liver cancer HepG2 cell proliferation was equally inhibited by the ethanolic leaf extract [73]. Bioactive compounds which include elagic acid, castalagin, and flavogallonic acid from A. leiocarpus, have been demonstrated to inhibit the proliferation of cancer cells in vitro [74]. Methanol extract of A. leiocarpus leaves inhibited cholinesterase activity while the tyrosinase activity was suppressed by a methanol extract of the stem bark [38].
The effects of acetic acid-induced ulcerative colitis in rats were studied in relation to A. leiocarpus leaf aqueous extract. The aqueous extract of A. leiocarpus leaves exhibited anti-colitis actions by increasing superoxide dismutase (SOD) and catalase (CAT) levels, decreasing glutathione (GSH) levels, and elevating superoxide dismutase (GSH) levels while lowering MDA (Malonidialdehyde) and NO (Nitric oxide) levels. The extract preserved normal haematological parameters and treated inflammation brought on by acetic acid at doses of 100 and 200 mg/kg [75,76].
In a recent study involving acid-induced writhing in Wistar rats’ model, the antinociceptive and antipyretic properties of A. leiocarpus aqueous leaf extract were examined. The extract was also assessed for safety using the median lethal dose (LD50). The extract significantly (p < 0.05) reduced/eliminated the induced pain and pyrexia at doses of 200 and 400 mg/kg in a way that was equivalent to the positive controls. A. leiocarpus aqueous leaf extract reportedly possesses antinociceptive and antipyretic properties [77].
A. leiocarpus stem bark extract significantly modifies the activities of phosphodiesterase-5, arginase, and acetylcholinesterase in male rats receiving paroxetine treatment thereby altering sexual behaviour and boosting antioxidant status, as well as biomolecules such as total thiol, malondialdehyde, nonprotein thiol and nitric oxide levels. These actions indicate some potential mechanisms that may underline their application in the treatment of erectile dysfunction induced by antidepressants [78,79,80]. A. leiocarpus extract has a pro-fertility effect. As a result, it serves as a good alternative for treating male infertility [ 80].
Conclusion
A. leiocarpus, a ubiquitous plant in the tropical woodlands and savannas is a multi-medicinal plant. Its folkloric applications which include the management of cough, wounds, stomach infections, tuberculosis, diarrhoea, and malaria make it highly desirable. Its other applications in the management of erectile dysfunction, antimicrobial, antibacterial, anticancer, anti fungal, antioxidant, antinociceptive and antipyretic, anti-plasmodial activities among others makes it a target plant for more extensive investigations. Alkaloids, tannins, terpe noids, flavonoids, cardiac glycosides, and saponins are the secondary metabolites that have been found in A. leiocarpus. While the compounds identified in the plant include gentisic, gallic acids, chebulagic acid, bislactone, castalagin, catechin, quercetin and some others, many more chemical compounds are yet to be identified and characterized particularly from the root, wood, fruit and flower which have been grossly under explored. The increasing demand for more potent antimicrobial agents makes the investigation of important underexplored folkloric medicinal plant such as A. leiocarpus more imperative particularly for the discovery of a drug lead. Future work should focus on the establishment of the possible mechanism of action of the identified compounds, discovery of potential drug leads and establishment of the toxicity of the extracts and constituent compounds. Apparently, more robust in vivo and holistic clinical studies are necessary to fully validate the traditional claims on the plant.
Conflict of Interest
The authors declare that there is no conflict of interest.
References
- Cragg GM, Newman DJ. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta, Gen. Subj., 2013, 1830(6), 3670-3695.
- Salih EYA, Kanninen M, Sipi M, Luukkanen O, Hiltunen R, Vuorela H, Julkunen-Tiitto R, Fyhrquist P. Tannins, flavonoids and stilbenes in extracts of African savanna wood-land trees Terminalia brownii, Terminalia laxiflora and Anogeissus leiocarpus showing promising antibacterial potential. S. Afr. J. Bot., 2017, 108, 370-386.
- Akande A, Ahmad M, Majid U. A qualitative meta-synthesis on how autonomy promotes vaccine rejection or delay among health care providers. Health Promot. Int., 2022, 37(1), daab099.
- Adenıjı O, İrunokhai E, Adigun J, Olorunfemi S. Diversity and abundance of tree species of a protected woodland : Southern Guinea savanna zone (Nigeria). Turk. J. Biodiversity, 2021, 4(2), 69-76.
- Adenıjı O, Zaccheaus OS, Ojo BS, Adedeji AS. Charcoal production and producers’ tree species preference in Borgu Local Government Area of Niger State, Nigeria. J. Energy Technol. Policy, 2015, 5(11), 1-8.
- Niass O, Diop A, Samb I, Diop A, Dieng A, Diop YM. Antibacterial and antifungal activity essay of alkaloids extracted from four Combretaceae used in Senegal. Int. J. Chem., 2022, 14(2), 59-62.
- Elufioye TO, Williams BM, Cyrl-Olutayo MC. Identification of the anti-sickling activity of Anogeissus leiocarpus and in silico investigation of some of its phytochemicals. Avicenna J. Med. Biochem., 2020, 8(1), 1-14.
- Muhammad HA, Yusha’u M, Taura DW, Aliyu AM. Antibacterial activity and phytochemical constituents of African birch (Anogeissus leio-carpus) stem bark extracts. Bayero J. Pure Appl. Sci., 2022, 13(1), 447-454.
- Agada JO, Gberikon GM, Amuta, EU. Antibacterial Actions of Anogeissus leiocarpus and Morinda lucida leaves, stems and roots extracts against some enteric bacteria. Int. J. Innovative Res. Adv. Stud., 2019, 6(9), 160-163.
- Dayok O, Dawang DN , Da’a m CE . Antimicrobial activity of leaf extract of Anogeissus leiocarpus (African birch) on some selected clinical isolates. IOSR J. Pharm. Biol. Sci., 2018, 13(4), 36-40.
- Audu BS, Wakawa IA, Oyewole OJ, Changdaya PZ. Histopathological alterations in the gills and liver of Clarias gariepinus juveniles exposed to acute concentrations of Anogeissus leiocarpus. Jordan J. Biol. Sci., 2021, 14(3), 537-543.
- Arbab AH. Review on Anogeissus leiocarpus a potent African traditional drug. Int. J. Res. Pharm. Chem., 2014, 4(3), 496-500.
- Singh D, Baghel US, Gautam A, Baghel DS, Yadav D, Malik J, Yadav R. The genus Anogeissus: A review on ethnopharmacology, phytochemistry and pharmacology. J. Ethnopharmacol., 2016, 194, 30-56.
- Ezuruike UF, Prieto JM. The use of plants in the traditional management of diabetes in Nigeria: Pharma-cological and toxicological considerations. J. Ethnopharmacol., 2014, 155(2), 857-924.
- Ouédraogo DY, Mortier F, Gourlet‐Fleury S, Freycon V, Picard N. Slow‐growing species cope best with drought: Evidence from long‐term measurements in a tropical semideciduous moist forest of Central Africa. J. Ecol., 2013, 101(6), 1459-1470.
- Klaus C, Plaimauer B, Studt J-D, Dorner F, Lämmle B, Mannucci PM, Scheiflinger F. Epitope mapping of ADAMTS13 autoantibodies in acquired thrombotic thrombocytopenic purpura. Blood, 2004, 103(12), 4514-4519.
- Abubakar U, Yusuf K, Abdu G, Saidu S, Jamila G, Fatima A. Ethnopharmacological survey of medicinal plants used for the management of pediatric ailments in Kano State, Nigeria. Res. J. Pharmacogn., 2017, 4(3) 29-39.
- Awobode HO, Fagbemi FT, Afolayan FID. Antitrypanosomal activity of Khaya senegalensis and Anogeissus leiocarpus stem bark on Trypanosoma brucei brucei infected rats. Afr. J. Biotechnol., 2015, 14(6), 525-529.
- Tauheed AM, Mamman M, Ahmed A, Suleiman MM, Balogun EO. In vitro and in vivo antitrypanosomal efficacy of combination therapy of Anogeissus leiocarpus, Khaya senegalensis and potash. J. Ethnopharmacol., 2020, 258.
- Ekhuemelo DO, Onuche MU, Tembe ET. Crude extracts of Eucalyptus camaldulensis (Dehnh) leaves and bark on Anogeissus leiocarpus (DC.) Guill. & Perr. and Daniellia oliveri Rolfe, hutch wood in control of termite (Isoptera: Termitidae). Int. J. Sci. Eng. Invest., 2017, 6, 66.
- Akanbi OM, Omonkhua AA, Cyril-Olutayo MC. Effect of methanolic extract of stem bark of Anogeissus leiocarpus on liver function of mice infected with Plasmodium berghei. J. Herbs, Spices Med. Plants, 2014, 20(4), 350-358.
- Shuaibu MN, Wuyep PT, Yanagi T, Hirayama K, Ichinose A, Tanaka T, Kouno I. Trypanocidal activity of extracts and compounds from the stem bark of Anogeissus leiocarpus and Terminalia avicennoides. Parasitol. Res., 2008, 102(4), 697-703.
- Salih EY, Julkunen-Tiitto R, Luukkanen O, Sipi M, Fahmi MK, Fyhrquist PJ. Potential anti-tuberculosis activity of the extracts and their active components of Anogeissus leiocarpa (DC.) Guill. and Perr. with special emphasis on polyphenols. Antibiotics, 2020, 9(7), 364.
- Belemnaba L, Ouédraogo S, Nitiéma M, Chataigneau T, Guissou IP, Schini-Kerth VB , Bucher B, Auger C. An aqueous extract of the Anogeissus leiocarpus bark (AEAL) induces the endothelium-dependent relaxation of porcine coronary artery rings involving predominantly nitric oxide. J. Basic Clin. Physiol. Pharmacol., 2018, 29(6), 599-608.
- Sombié BC, Claude J, Ouédraogo W, Nitiema M. Standardization of extracts from trunk 's barks of Lannea macrocarpa Engl. and K. Krause (Anacardiaceae) and Anogeissus leiocarpus (DC.) Guill. and Perr. (Combretaceae) for the formulation of antihypertensive herbal medicines. Int. J. Pharm. Sci. Rev. Res., 2018, 48(1), 92-97.
- Konan KS, Tiekpa WJ, Moroh AJ-L. Anticoccidial activity of the aqueous extract of Anogeissus leiocarpus leaves in broiler chickens. J. Pharmacogn. Phytochem., 2020, 9(6), 41-44.
- El Ghazali G, Adam I, Hamad A, El Bashir MI. Plasmodium falciparum infection during pregnancy in an unstable transmission area in eastern Sudan. East. Mediterr. Health J., 2003, 9(4), 570-580.
- Okpekon T, Yolou S, Gleye C, Roblot F, Loiseau P, Bories C , Grellier P, Frappier F, Hocquemiller R. Anti parasitic activities of medicinal plants used in Ivory Coast. J. Ethnopharmacol., 2004, 90(1), 91-97.
- Batawila K, Kokou K, Koumaglo K, Gbéassor M, de Foucault B, Bouchet P, Akpagana, K. Antifungal activities of five Combretaceae used in Togolese traditional medicine. Fitoterapia, 2005, 76(2), 264-268.
- Barku VY, Boye A, Ayaba S. Phytochemical screening and assessment of wound healing activity of the leaves of Anogeissus leiocarpus. Eur. J. Exp. Biol., 2013, 3(4), 18-25.
- Timothy SY, Mashi FI, Helga BI, Galadima IH, Midala TAS. Phytochemical screening, antibacterial evaluation and in vitro spasmodic effect of the aqueous and ethanol leaf and bark extracts of Anogeissus leiocarpus (DC.) Guill. & Perr. Asian J. Pharm. Sci. Technol., 2015, 5(4), 302-308.
- Osuntokun OT, Oluwafoise BO. Phytochemical screening of ten Nigerian medicinal plants. Int. J. Multidiscip. Res. Dev., 2015, 2(4), 390-396.
- Dahiru MM, Badgal EB, Musa N. Phytochemical profiling and heavy metals composition of aqueous and ethanol extracts of Anogeissus leiocarpus. Ankara Univ. Eczacilik Fak. Derg., 2022, 47(2), 1-13.
- Ezeonu CS, Ejikeme CM. Qualitative and quantitative determination of phytochemical contents of indigenous Nigerian softwoods. New J. Sci., 2016, 2016, 1-9.
- Namadina MM, Mukhtar AU, Karaye SI, Musa FM, Bah IH, Maitama FY. Phytochemical constituents and antibacterial activities of indigenous chewing stick (Anogeissus leiocarpus) stem. Bayero J. Pure Appl. Sci.,2021, 14(1), 85-94.
- Ogunjobi KM, Abdulwahab SO, Gakeno OF, Thompson OE, Olorunfemi O. Qualitative and quantitative evaluation of the phytochemical constituents of three wood species in Ogun state, Nigeria. Trop. Plant Res., 2020, 7(3), 627-633.
- Hussaini Y, Bello RY, Mustapha T. Preliminary phytochemical screening and GC-MS analysis of Anogeissus leiocarpus stem bark extract. Pharm. Innovation J., 2022, 11(11), 113-117.
- Orlando UD, Castillo AF, Medrano MAR, Solano AR, Maloberti PM, Podesta EJ. Acyl-CoA synthetase-4 is implicated in drug resistance in breast cancer cell lines involving the regulation of energy-dependent transporter expression. Biochem. Pharmacol., 2019, 159, 52-63.
- Chaabi M, Benayache S, Benayache F, N'Gom S, Koné M, Anton R , Weginer B, Lobstein A. Triterpenes and polyphenols from Anogeissus leiocarpus (Combretaceae). Biochem. Syst. Ecol., 2008, 36(1), 59-62.
- Hamzaoui M, Renault J-H, Nuzillard J-M, Reynaud R, Hubert J. Stepwise elution of a three‐phase solvent system in centrifugal partition extraction: A new strategy for the fractionation and phytochemical screening of a crude bark extract. Phytochem. Anal., 2013, 24(4), 367-373.
- Agaie BM, Onyeyili PA. In vitro anthelmintic activity of the aqueous leaf extract of Anogeissus leiocarpus and its phytochemical, proximate and elemental contents. J. Med. Plants Res., 2011, 5(30), 6656-6661.
- Ogundele G, Salawu S, Abdulraheem I, Bamidele O. Nutritional composition of oyster mushroom (Pleurotus ostreatus) grown on softwood (Daniella oliveri) sawdust and hardwood (Anogeissus leio-carpus) sawdust. Br. J. Appl. Sci. Technol., 2017, 20(1), 1-7.
- Aburjai T, Darwish RM, Al-Khalil S, Mahafzah A, Al-Abbadi A. Screening of antibiotic resistant inhibitors from local plant materials against two different strains of Pseudomonas aeruginosa. J. Ethnopharmacol., 2001, 76(1), 39-44.
- Salau AK, Yakubu MT, Oladiji AT. Effects of aqueous root bark extracts of Anogeissus leiocarpus (DC.) Guill. & Perr. and Terminalia avicennioides Guill. & Perr. on redox and haematological parameters of diethylnitrosamine-administered rats. Iran. J. Toxicol., 2018, 10(1),21-29.
- Eltayeb IM, Ali HAR, Muddather AK, Ayoub SMH . Antioxidant activity and cytotoxic studies of Anogeissus leiocarpous root, leaf and stem. Am. J. Res. Commun., 2016, 4(3), 52-57.
- Onoja US, Ugwu CC, Uzor PF, Nweze IE, Omeje EO, Nnamani PO, Nwachukwu E, Njoku I, Effiong EJ. Effect of Anogeissus leiocarpus Guill. and Perr. leaf on hyperglycaemia and associated dyslipidaemia in alloxan-induced diabetic rats. Dhaka Univ. J. Pharm. Sci., 2018, 17(1), 65-72.
- Idakwoji PA, Agatemor UM-M , Akuba BO, Oniemola JM, Momoh TB. Effect of aqueous leaf extract of Anogeissus leiocarpus against experimental models of pain and pyrexia. Screening of medicinal plants for anti-diabetic properties (View project). Molecular identification, structure elucidation and characterization of bioactive prin-ciples in medicinal plants using FTIR, GC-MS, UPLC-PDA-QTOF-ESI-MS/MS and NMR techniques: Investigation of in vitro and in vivo bioactivities (View project). Article in Int. J. Adv. Res. Biol. Sci., 2019, 6(6), 181-188.
- Motto, AE, Lawson-Evi P, Kantati Y, Eklu-Gadegbeku K, Aklikokou K, Gbeassor M. Antihyperglycemic activity of total extract and fractions of Anogeissus leiocarpus. J. Drug Delivery Ther., 2020, 10(3), 107-113.
- Motto AE, Lawson-Evi P, Eklu-Gadegbeku K. Antidiabetic and antioxidant potential of total extract and supernatant fraction of the roots of Anogeissus leiocarpus in HFD-fed and Streptozocin-induced diabetic rats. Biomed. Pharmacother., 2022, 154, 113578.
- Ogbuagu NE, Aluwong T. Antidiabetic and antioxidant activities of ethanolic extract of Anogeissus leiocarpus in alloxan-induced type 1 diabetes mellitus in Wistar rats. Cronicon Ec Diabetes Metab. Res., 2022, 6.1, 38-49.
- Adefegha SA, Oboh G, Omojokun OS, Jimoh TO, Oyeleye SI. In vitro antioxidant activities of African birch (Anogeissus leiocarpus) leaf and its effect on the α-amylase and α-glucosidase inhibitory properties of acarbose. J. Taibah Univ. Med. Sci., 2016, 11(3), 236-242.
- Num-Adom SM, Adamu S, Aluwong T, Ogbuagu NE, Umar IA, Esievo KAN. Ethanolic extract of Anogeissus leiocarpus ameliorates hyperglycaemia, hepato-renal damage, deranged electrolytes and acid-base balance in alloxan-induced diabetes in dogs. Sci. Afr., 2022, 16.
- Omeje E, Osadebe P, Obonga W, Ugwuoke C. Phytochemical and pharmacological basis for the ethnomedicinal use of root extracts from Anogeissus leiocarpus as an antidiabetic in Eastern Nigeria. Br. J. Pharm. Res., 2016, 9(4), 1-8.
- Magashi LA, Nuhu I. Antimicrobial activity of the roots and stem bark of Ceiba pentandra and Anogeissus leiocarpus grown in Bauchi, North Eastern Nigeria. J. Pharmacogn. Phytochem., 2017, 6(5), 865-870.
- Ali EO, Tor-Anyiin TA, Hammuel C. Antimicrobial activity of Anogeissus leiocarpus stems bark extracts and an isolate from the plant against some microbes. Am. J. Res. Commun., 2017, 5(8), 9-25.
- Gbadamosi IT, Ogunsuyi AO. An appraisal of the potency of roots of Anogeissus leiocarpus (DC.) Guill. & Perr. and Terminalia glaucescens Benth. in the management of E. coli related infections. J. Appl. Biosci., 2014, 78, 6646-6653.
- Lawal TO , Bamiduro TB , Ofonmbuk JM , Elufioye TO , Adeniyi BA , Mahady GB . Antibacterial effects of Anogeissus leiocarpus (DC.) Guill. & Perr. and Terminalia glaucescens Planch. Ex Benth. on rapidly growing mycobacteria species. Afr. J. Microbiol. Res., 2017, 11(12), 495-503.
- Harisu Y U, Muhammad AI. Antibacterial activity of leaves extract of Anogeissus leiocarpus and Vitex doniana against some bacteria. Control of malaria (View project). Study of Biochemical and Hematological Markers in Malaria-Malnutrition Co-Morbidity among Children Under 5 Years of Age in Kano State Nigeria (View project). Am. J. Innovative Res. Appl. Sci., 2015, 1(10), 284-288.
- Edewor TI, Akpor OB, Owa SO. Determination of antibacterial activity, total phenolic, flavonoid and saponin contents in leaves of Anogeissus leiocarpus (DC.) Guill. and Perr. J. Coastal Life Med., 2016, 4(4), 310-314.
- Elsiddig IME, Muddather AK, Ali HAR, Ayoub SMH. A comparative study of antimicrobial activity of the extracts from root, leaf and stem of Anogeissus leiocarpous growing in Sudan. J. Pharmacogn. Phytochem., 2015, 4(4), 107-113.
- Mann A, Yusuf A, Daniyan S. TLC analysis and bioactivity screening of the stem bark extract of Anogeissus leiocarpus against multi-resistant Staphylococcus aureus and quantification of its phytoconstituents. Screening of Indigenous Plants with Hypoglycemic and Antimicrobial Potentials (View project). Evaluating Effects of feeding detoxified Jatropha curcas meal and oil on the survival, growth and body compositions of Clarias gariepinus (View project). Res. J. Pharm. Biol. Chem. Sci., 2014, 5(2), 187-203.
- Muhammad FJ, Yafu MA, Yakubu M, Enemaku OF, Anthonia AB. Nigerian plants with anti-inflammatory and antifungal potential. J. Adv. Biopharm. Pharmacovigilance, 2019, 1(2), 40-48.
- Maigari AK, Mukhtar Y Abubakar A, Muhammad B, Adamu A, Malami AI, Baura MS, Yaro MA, Harazimi AC, Umar RB, Barkindo MA. Antifungal activities of Anogeissus leiocarpus (African birch) against Aspergillus niger and Rhizoctonia species. Int. J. Health Pharm. Res., 2017, 3(2), 30-39.
- Elsiddig IME, Muddathir, AK, Ali HAR, Ayoub SMH. In vitro susceptibility of Madurella mycetomatis to the extracts of Anogeissus leiocarpus leaves. Int. J. Biol. Biomolec. Agric. Food Biotechnol. Eng., 2015, 9(12), 1039-1050.
- Mann A, Banso A, Clifford LC. An antifungal property of crude plant extracts from Anogeissus leiocarpus and Terminalia avicennioides. Tanzania J. Health Res., 2008, 10(1), 34-38.
- Gara TY. In-vitro activity of methanolic extract of stem bark of Anogeisuss leiocarpus (African birch) on Plasmodium falciparum. Int. J. Appl. Biol. Res., 2020.
- Akanbi OM, Omonkhua AA, Cyril-Olutayo CM, Fasimoye RY. The antiplasmodial activity of Anogeissus leiocarpus and its effect on oxidative stress and lipid profile in mice infected with Plasmodium bergheii. Parasitol. Res., 2012, 110(1), 219-226.
- Akanbi O. Antiplasmodial potential of combination therapy of methanolic bark extracts of Terminalia avicennioides and Anogeissus leiocarpus and its effect on haematological parameters on mice infected with Plasmodium berghei. J. Adv. Med. Pharm. Sci., 2017, 15(2), 1-9.
- Tagne MAF, Rékabi Y, Noubissi PA, Fankem GO, Akaou H, Wambe H, Kamgang, R. Evaluation of antidiarrheal activity of aqueous leaf extract of Anogeissus leiocarpus on castor oil-induced diarrhea in rats. Am. J. Biomed. Sci. Res., 2019, 3(1), 27-34.
- Irinmwinuwa EO, Adolphus MC, Ibeabuchi KC, Benard GO. Evidence based medicinal plant possessing anti-diarrhea activity: A review. Natl. J. Adv. Res., 2023, 9(1), 1-6.
- Hassan LEA, Al-Suade FS, Fadul SM, Abdul Majid AMS. Evaluation of antioxidant, antiangiogenic and antitumor properties of Anogeissus leiocarpus against colon cancer. J. Angiother., 2018, 1(2), E056–E066.
- Olugbami JO, Damoiseaux R, France B, Onibiyo EM, Gbadegesin MA, Sharma S , Gimzewski JK, Odunola OA. A comparative assessment of antiproliferative properties of resveratrol and ethanol leaf extract of Anogeissus leiocarpus (DC.) Guill. and Perr. against HepG2 hepatocarcinoma cells. BMC Complementary Altern. Med., 2017, 17(1), 1-11.
- Ukwade CE, Ebuehi OAT, Adisa RA. Phytochemical and cytotoxic screening of selected medicinal plants (Byrsocarpus coccineus, Terminalia avicennioides and Anogeissus leiocarpus) using brine shrimp (Artemia salina) lethality assay. Eur. J. Nutr. Food Saf., 2020, 60-71.
- Ohiagu FO, Chikezie PC, Chikezie CM, Enyoh CE. Anticancer activity of Nigerian medicinal plants: A review. Future J. Pharm. Sci., 2021, 7(1), 1-21.
- Tagne MAF, Noubissi PA, Gaffo EF, Fankem GO, Mukam JN, Kamgang R, Oyono J -LE Effects of aqueous extract of Anogeissus leiocarpus (DC.) Guill. Et Perr. (Combretaceae) leaves on acetic acid‐induced ulcerative colitis in rats. Adv. Tradit. Med., 2021, 1-10.
- Rufa’i H, Nzelibe HC, Musa AM. Gastroprotective effect of aqueous stem bark extract of Anogeissus leiocarpus against ethanol-induced gastric ulcer in rats. Sci. World J., 2022, 17(1), 11-16.
- Idakwoji PA, Atanu FO, Nweje-Anyalowu PC, Momoh TB, Oniwon WO, Elazab ST , Sharkawi SMZ, Waheed RM, Youssef A, Batiha GE-S. Pharmacological studies of anti-inflammatory, anti-nociceptive and anti-pyretic compounds found in chromatographic fractions of Anogeissus leiocarpa (DC). Guill. & Perr. leaves. J. Pharm. Pharmacogn. Res., 2022, 10(3), 459-468.
- Ademosun AO, Adebayo AA, Oboh G. Anogeissus leiocarpus attenuates paroxetine-induced erectile dysfunc-tion in male rats via enhanced sexual behavior, nitric oxide level and antioxidant status. Biomed. Pharma-cother., 2019, 111, 1029-1035.
- Oboh G, Adebayo AA, Ademosun AO, Boligon AA. In vitro inhibition of phosphodiesterase-5 and arginase activities from rat penile tissue by two Nigerian herbs (Hunteria um-bellata and Anogeissus leiocarpus). J. Basic Clin. Physiol. Pharmacol., 2017, 28(4), 393-401.
- Ahmed OM, Ozikio EN, Agatemor UM-M Olubiyo GT, Momoh TB. Pro-fertility effect of the aqueous leaf extract of Anogeissus leiocarpus in albino Wistar rats. Pharmacological and Toxicological Profiling of Common Polyherbal Formulations (View project). Neuropharmacological Pro-file of Leptadenia hastata (View project). Int. J. Curr. Res. Med. Sci., 2020, 6(5), 1-6.
|