|
Introduction Currently, use of phytochemicals, which are derived from medicinal plants, has gradually increased in many countries [1]. Medicinal plants have a wide variety of chemical compounds, resulting in different types of medicines and various bioactive molecules [2]. According to the World Health Organization (WHO), medicinal plants will be the best source for a variety of medicines and natural antioxidants, as they have long played important roles in the treatment of diseases throughout the world [1]. Some of the properties that the medicinal plants have are antimicrobial, anti-cancer, anti-diabetic, anti-atherosclerosis, and immunomodulatory, and even have reno-protective or hepato-protective effects [3]. Figs (Ficus carica L.), mulberry (Morus alba L.) and eucalyptus (camaldulensis L.) are among these medicinal plants whose aqueous extracts are of great importance because they contain many important biochemical compounds. The fig plant has a wide range of medicinal and nutritional values [4]. Its fruit, roots and leaves are used in native medicine for different disorders, such as colic, indigestion, diarrhea, sore throats, coughs, bronchial problems, inflammatory and cardiovascular disorders, ulcerative diseases, and cancers [5]. Clinical studies have shown that fig leaves extract has anti-tumor, hypolipidemic, antioxidant, antibacterial, hypoglycemic, and other functions. Because fig leaves contain a large number of flavonoids, they have a variety of pharmacological activities [4]. Mulberry trees are deciduous plants belonging to the genus Morus (family Moraceae), The most common species are Morus alba (white mulberry) and Morus nigra (blackberry) [6]. Morus alba L. leaves have an antiparasitic activity [7] and contain active ingredients showing a high nutritional importance and pharmaceutical effects among the genus Morus. They have long been used as traditional medicine for diabetes, arthritis, rheumatism, and other disorders for thousands of years in East Asia [6]. Eucalyptus (camaldulensis L.) is one of the important genera of the Myrtaceae family, a large genus of evergreen trees; it has been used as a medicinal plant for ages because of its various properties [8]. Eucalyptus’ therapeutic properties include antiseptic, antiparasitic, insect repellent, anti-rheumatism, anti-migraines, anti-urinary tract infections, anti-ulcer burns, febrifuge, and anti-fatigue [9]. The present study was conducted to investigate the effects of fig (Ficus carica L.), mulberry (Morus alba L.) and eucalyptus (camaldulensis L.) leaves extracts on the germination and growth of maize (Zea mays L.) seeds due to it being one of the most economically important food crops in the world. It possesses high nutritive value and is important as a coarse grain. Germination is the first stage and one of the important and sensitive stages of the plant life cycle; it is an important process in seedling growth. This stage of growth is strictly influenced by environmental factors [10]. Additionally because these medicinal plant leaves are widely used in multiple fields, it was important to identify their chemical constituents.
Materials and Methods This study was conducted in the laboratories of Syrian Private University, Syria, in 2022. Preparation of Plant The plant materials, fig (Ficus carica L.), mulberry (Morus alba L.) and eucalyptus (camaldulensis L.) leaves, were collected from the experimental fields of Tishreen Park, Damascus City, Syria in June 2022. The experiments were performed in a completely randomized design with three replications.The seeds of maize (Zea mays L. Ghouta 82) were obtained from the General Institution for Plenitude of Seeds (Aleppo - Syria). Preparation of Extracts Fresh leaves of fig, mulberry and eucalyptus plants were picked and cleared of any foreign materials, then rinsed with distilled water and dried with absorbent paper for 15 days in the shade. They were then grinded with an electric grinder to a fine powder that was used for the extraction [11]. Extraction was done using the method described by Dzimitrowicz et al. [12] with some modification. Twenty grams of fine powder of each plant were mixed separately in 400 mL of distilled water for one hour at room temperature with continuous stirring by a magnetic stirrer. The extracts were heated to 60°C for one hour, and after, were gradually cooled with continuous stirring until room temperature was reached, then left to stand for 24 h at 4°C. The resulting aqueous plant solutions were filtered twice with multi-layer tissue, then with Buchner funnels containing (Whatman® filter paper no. 5) and connected to a vacuum pump. Finally, the filtrates of the aqueous plant extracts were stored in the dark at 4°C until further used in bioassay and phytochemical characterization. Figure 1 shows the dried plants leaves before and after grinding, as well as the plant extract.
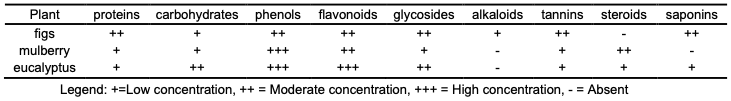
Qualitative Analysis Preliminary qualitative phytochemical screening was carried out for proteins (Biuret’s test) and glycosides (Salkowski’s test) [13], carbohydrates (Molisch’s test) [14], phenols [15,16], flavonoids (Alkaline reagent test) and alkaloids (Mayer’s test) [17], tannins (Ferric chloride test) [18], steroids (Lieberman-Burchard test) [15,17], and saponins (Frothing test) [19], following the standard protocols.
Maize (Zea mays L.) Seeds Germination Experiment For bioactivity study, healthy maize seeds of uniform size were used for the experiment. Surfaces were sterilized with 0.1% NaOCl, then they were washed thoroughly with distilled water [20]. The seeds were soaked for 24 h at room temperature (24°C) in different concentration of fig, mulberry and eucalyptus leaves extracts (5, 10, 15, 25, 50 and 75% v/v), using the distilled water as the control, then allowed to germinate on moist paper towels in petri dishes at 37°C in darkness for six days (144 h). The experimental seeds were kept moist by regularly adding of test solution, if required [21,22]. The germination percentage is the propor- tion, expressed as percentage of germinated seeds to the total number of viable seeds that were tested by the following formula according to ISTA [23]. The seedling growth was harvested after six days. The root length and shoot length were measured by using a centimeter scale, root length was measured from the main apex to the crown, whereas shoot length was mea- sured from the crown to the main apex [24].
Statistical Analyses The data was subjected to one way-ANOVA IBM SPSS software package for Windows (Version 20, SPSS Inc., Chicago, IL), the statistical significance was evaluated at P≤ 0.05. The results were presented as mean ± standard deviation based on three replications.
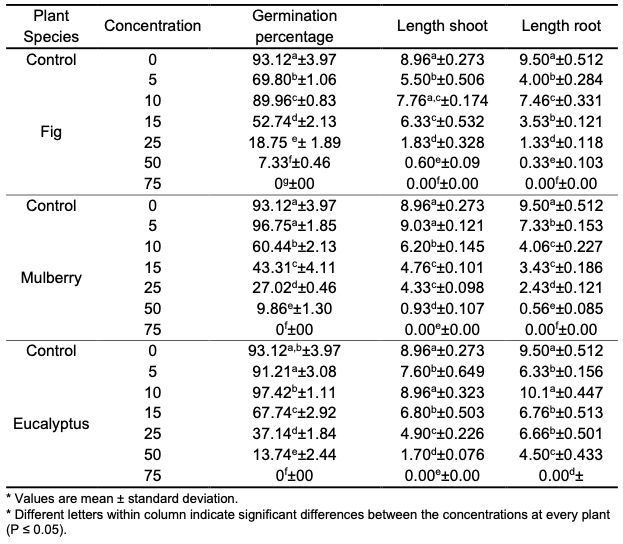
Results and Discussion Effects of the different concentrations (5, 10, 15, 25, 50 and 75% v/v) of fig, mulberry and eucalyptus leaves extracts were studied on germination percentage of maize seeds (Zea mays L.); distilled water was used as the control. Results showed that seed germination of maize was promoted or inhibited to different degrees depending on the concentrations of aqueous extracts. This indicates that maize has a strong adaptability, which plays a pivotal role in it becoming a dominant species. At low concentrations of plant extracts (5%), the mulberry extract showed the highest germination percentage of 96.75%, followed by eucalyptus extract with a germination percentage close to that of the control (91.21%), while the fig extract at the same concentration reached 69.80%. By increasing the concentration of all plant extracts to 10%, a significant improvement and significant differences were observed for each of the extracts of fig and eucalyptus compared to the previous concentration, where the percentage of germination reached 89.96% and 97.42%, respectively. The percentage of germination of mulberry extract decreased to 60.44% with clear significant differences compared to the control and at the previous concentration. This may be due to the increased osmotic pressure of plants cells, which increases the water absorption capacity of cells. It may also be that the trace inorganic ions in maize cells have a stimulating effect on respiratory enzyme activity, which improves the ability of plants to generate nutrients, therefore promoting seed germination of maize [25]. Allelochemicals are usually called secondary plant products of the main metabolic pathway in plants, most of them originate from the shikimic acid and acetate pathway. They are often water-soluble substances and present in almost all plants and in many tissues. Allelochemicals that inhibit the growth of some species at certain concentrations might in fact stimulate the growth of the same or different species at different concentrations [26]. The increase in the concentrations of the plant extracts led to a clear decrease in the germination percentage of maize seeds. At the concentration of 15%, the germination percentage of figs, mulberry and eucalyptus was 52.74% and 43.31% and 67.74%, respectively, and at the concentration of 25% was 18.75% and 27.02% and 37.14%, respectively. The inhibition in the ability to germinate was more evident at the 50% concentration, where the percentage of germination was 18.75% and 27.02% and 37.14% for each of the extracts of figs, mulberry and eucalyptus, respecttively. While the germination and growth of maize seeds were completely inhibited at 75% concentration. The allelochemical content increased with the increase of the concentrations of the studied plants leaves aqueous extracts, resulting in an enhanced inhibition effect. Certain plant allelochemicals have hormone-like effects or promote the growth of the recipient plant by changing its hormone composition and concentration [25]. Table 1 shows the germination percentage of maize seeds, and root and shoot lengths of maize seedling when using distilled water as a control and at different concentrations of extracts of leaves of fig, mulberry and eucalyptus.
By studying the effect of different concentrations of extracts of leaves of fig, mulberry and eucalyptus plants on the growth of seedlings of maize plant, the change in shoot and root length was recorded with the change in the concentration of each of the extracts. The results showed that the shoot length under the influence of each of the extracts of fig and eucalyptus leaves ranged between 7.76 cm and 0.60 cm for fig and between 8.96 cm and 1.70 cm for eucalyptus, and the length of the root ranged between 7.46 cm and 0.33 cm for fig and between 10.1 cm and 4.50 cm for eucalyptus, where these values were recorded at concentration 10% and 50%, respectively. Whereas, with the effect of mulberry leave extract, it was observed that the highest growth of shoot and root was recorded at the concentration 5% where the length of each of the shoot and root was 9.03 cm and 7.33 cm, respectively. From the above, it is noted that the toxicity of fig and eucalyptus extracts on both the shoot and root started at a concentration of 15%, while the toxicity of the mulberry extract started at 10%, and this toxicity increased with increasing concentration. Germination and seedling growth are the screening criteria which are widely used to investigate the effects of allelopathy. Morphological changes, in response to allelochemicals, could be due to effects on the cellular or molecular level. The effects of the allelochemicals’ action have been detected at molecular, structural, physiological, biochemical and ecological levels of plant organization. Allelochemicals restrict plant growth through negative interactions with some physiological processes such as suppression of cell division, changes in cell wall structure and activity of some enzymes [27]. Also, the effect of allelopathy on germination and growth of plants may occur through a variety of mechanisms, including a reduced mitotic activity in root and hypocotyls, suppressed hormone activity, reduced rate of nutrient uptake, inhibited protein formation, decreased permeability of cell membranes and inhibition of enzyme action which may be attributed to the reduction of (N, P, K) content in the tested seeds of maize. The tested seeds differed in their responses, which may be due to the effect of the extracts on the cell permeability to the nutrient’s uptake, or the genetic effect, because the inhibitory compounds might have reduced the uptake of nutrient, which ultimately reduced shoot growth [28]. This study has revealed the presence of phytochemicals considered as active medicinal chemical constituents. Some of the phytochemicals were found in abundance while others in trace amounts. Table 2 showed preliminary phytochemical screening of the fig, mulberry and eucalyptus leaves aqueous extracts.
Phytochemical studies and qualitative phytochemical investigation discovered the presence of proteins, carbohydrates, phenols, flavonoids, glycosides and tannins in all mentioned extracts of plant. Steroids are found in all extracts except for those obtained from figs extract. Alkaloids were found in figs extract, while other plant extracts did not contain this type of compound. Saponins were not found in mulberry extract. Plant cells produce two types of metabolites. Primary metabolites are involved directly in growth and metabolism (carbohydrates, lipids and proteins). Secondary metabolites are considered products of primary metabolism and are generally not involved in metabolic activity (alkaloids, phenolics, essential oils and terpenes, sterols, flavonoids, lignins, tannins, etc.). These secondary metabolites are the major source of pharmaceuticals, food additives, fragrances and pesticides, and herbicides. The composition of bioactive compounds present in plants are influenced by the genotype, extraction procedure, geographic and climatic conditions, and the growth phase of the plants [15]. Saponins have anti-inflammatory and anti-fungal effects, hemolytic activity, and cholesterol binding properties. Tannins have been reported to prevent the development of microorganisms by precipitating microbial protein and making nutritional proteins unavailable for them. Also, tannins exhibit anti-oxidant and antiviral effects [15,18]. Steroids are known to produce an inhibitory effect on inflammation and are very important compounds, especially due to their relationship with compounds such as sex hormone [15,29]. Alkaloids have been reported to exert analgesic, antispasmodic and antibacterial activities [30]. Phenolic compounds like phenolic acids, polyphenols and flavonoids are very important plant components called antioxidants, which scavenge free radicals such as peroxide, hydroperoxide of lipid hydroxyl and therefore halt the oxidative mechanism that leads to degenerative diseases. Flavonoids have anti-inflammatory, anti-angiotic, antimicrobial, antioxidant, and reduced hypertension effects, and have anti-cholesterol properties [16]. The phytochemical analysis of the medicinal plants is also important and has commercial interest from both research institutes and pharmaceuticals companies for the manufacturing of the new drugs for treatment of various diseases.
Conclusion In the present study, the phytochemical screening for leaves extracts of figs, mulberry and eucalyptus showed the presence of active component like phenols, flavonoids, glycolsides and tannins from aqueous extracts. These extracts have an extremely strong effect on the seed germination of maize at different concentrations. The low concentrations of the leaves aqueous extracts of the studied plants showed an improvement in morphological growth and germination percentage, while the higher concentrations showed clear toxicity in the maize plant.
Funding This research received no external funding.
Conflict of Interest The manuscript was written through contributions of the two authors, and these two authors contributed equally. The authors have given approval to the final version of the manuscript. The authors declare no conflict of interest.
Acknowledgments The authors are thankful to the Syrian Private University and Atomic Energy Commission of Syria for providing the facility to conduct the experiments in laboratories.
References
|
|||||||||||||||||||||||||